Biohaven's BHV-1200, A Multimodal Antibody Therapy Enhancer (MATE), Demonstrates Effective Neutralization Of Multiple Strains Of COVID-19
Biohaven Pharmaceutical Holding Company (NYSE: BHVN) announced promising results for BHV-1200, a hyperimmune globulin mimic developed using its MATE platform, demonstrating its ability to neutralize SARS-CoV-2 and variants B.1.1.7 and B.1.351. Preliminary tests showed significant reduction in viral entry into cells. The development of this COVID-19 treatment has garnered support from the Bill and Melinda Gates Foundation. The CEO expressed excitement about advancing BHV-1200 into clinical trials, highlighting its potential advantages over existing therapies.
- BHV-1200 demonstrated effective neutralization of SARS-CoV-2 and its variants.
- Support from the Bill and Melinda Gates Foundation accelerates development.
- Potential advantages over convalescent plasma and monoclonal antibodies.
- None.
Insights
Analyzing...
NEW HAVEN, Conn., Feb. 22, 2021 /PRNewswire/ -- Biohaven Pharmaceutical Holding Company Ltd. (NYSE: BHVN), a commercial-stage biopharmaceutical company with a portfolio of innovative, late-stage product candidates, today announced that a hyperimmune globulin mimic (HGM) developed with Biohaven's proprietary MATE platform has demonstrated functional binding and neutralization of the SARS-CoV-2 virus, including the strains known as the "English" and "South African" variants (also known as B.1.1.7 and B.1.351, respectively). The preliminary experiments, conducted by Biohaven Labs and an academic collaborator demonstrated that BHV-1200 substantially reduced viral entry into cells. Accelerated development of the COVID-19 MATE program has been supported by the Bill and Melinda Gates Foundation.
Vlad Coric M.D., Chief Executive Officer of Biohaven commented, "The battle against COVID-19 –like the virus itself–is dynamic and ever changing. Prophylactic and therapeutic agents with broad specificity against current and future strains will be essential in this ongoing fight to beat the virus. Results from our recent preclinical testing are an exciting advancement for this platform technology, and shows that our lead novel MATE conjugate, BHV-1200, exhibits broad and potent antiviral activity against not only wild type SARS-CoV-2 spike protein but also against mutations of growing clinical relevance, such as those associated with reduced susceptibility to therapeutic monoclonal antibodies and recent SARS-CoV-2 strains." Dr. Coric added, "We believe that BHV-1200 could lead to enhanced efficacy and other benefits over convalescent plasma and alternative antibody-based approaches. Biohaven is excited to advance BHV-1200 into a full clinical development program. We are deeply grateful to the vision and critical funding support provided by The Bill & Melinda Gates Foundation that has accelerated this novel technology towards the clinic."
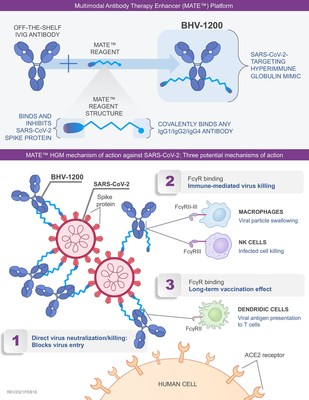
Biohaven's proprietary MATE conjugation technology uses a new class of synthetic peptide binders to target the spike protein of SARS-CoV-2 that are then selectively conjugated to commercially available intravenous immunoglobulin (see Figure 1). The Biohaven synthetic binders for SARS-CoV-2 were designed to establish a much wider area and number of contacts with the spike protein than other agents like monoclonal antibodies. Importantly, the binding and potent neutralizing activity observed with BHV-1200 was consistent across multiple strains of the SARS-CoV-2 virus, including the "English" and "South African" variants. These variants contain multiple mutations in the spike protein that have been reported to reduce the binding and neutralizing activity of currently available antibody-based COVID-19 therapies and sera from SARS-CoV-2 vaccine recipients. In addition, the in vitro data indicate that BHV-1200 may activate important immune system components including antibody-dependent cellular phagocytosis (ADCP) and antibody dependent cellular cytotoxicity (ADCC). Biohaven's proprietary MATE-conjugation technology could also be used against other infectious diseases by changing the targeting moiety of its antibody binders.
Dr. Charles Conway, Chief Scientific Officer at Biohaven stated, "Our recent in vitro data provide good evidence that BHV-1200 can neutralize the new strains of SARS-CoV-2 that have recently appeared across the globe. Our MATE-conjugation technology could enable commonly used and commercially available IVIG plasma products, which is not specific for the SARS-CoV-2 virus, to be redirected to target the viral spike protein. We look forward to continue to evaluate the therapeutic utility of this treatment both in the lab and in the clinic."
About Biohaven
Biohaven is a commercial-stage biopharmaceutical company with a portfolio of innovative, best-in-class therapies to improve the lives of patients with debilitating neurological and neuropsychiatric diseases, including rare disorders. Biohaven's neuroinnovation portfolio includes FDA-approved NURTEC® ODT (rimegepant) for the acute treatment of migraine and a broad pipeline of late-stage product candidates across three distinct mechanistic platforms: CGRP receptor antagonism for the acute and preventive treatment of migraine; glutamate modulation for obsessive-compulsive disorder, Alzheimer's disease, and spinocerebellar ataxia; and myeloperoxidase (MPO) inhibition for multiple system atrophy and amyotrophic lateral sclerosis. Biohaven Labs is a research and discovery arm of the company developing next-generation, bispecific compounds. More information about Biohaven is available at www.biohavenpharma.com.
Forward-Looking Statements
This news release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements involve substantial risks and uncertainties, including statements that are based on the current expectations and assumptions of the Company's management and the ability of BHV-1200 to produce enhanced efficacy following the full clinical development program. All statements, other than statements of historical facts, included in this press release regarding the Company's business and product candidate plans and objectives are forward-looking statements. Forward-looking statements include those related to the Company's strategies and objectives and its ability to achieve those objectives. The use of certain words, including "believe", "continue", "may", "on track", "expects" and "will" and similar expressions, are intended to identify forward-looking statements. Various important factors could cause actual results or events to differ materially from those that may be expressed or implied by our forward-looking statements. Additional important factors to be considered in connection with forward-looking statements are described in the "Risk Factors" section of Biohaven's Annual Report on Form 10-K filed with the Securities and Exchange Commission on February 26, 2020 and Biohaven's Quarterly Report on Form 10-Q for the quarter ended September 30, 2020 filed with the Securities and Exchange Commission on November 9, 2020. The forward-looking statements are made as of this date and Biohaven does not undertake any obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
NURTEC and NURTEC ODT are registered trademarks of Biohaven Pharmaceutical Ireland DAC.
MATE is a trademark of Kleo Pharmaceuticals, Inc. (a subsidiary of Biohaven and D/B/A Biohaven Labs)
Biohaven Contact
Dr. Vlad Coric
Chief Executive Officer
Vlad.Coric@biohavenpharma.com
![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/biohavens-bhv-1200-a-multimodal-antibody-therapy-enhancer-mate-demonstrates-effective-neutralization-of-multiple-strains-of-covid-19-301232221.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/biohavens-bhv-1200-a-multimodal-antibody-therapy-enhancer-mate-demonstrates-effective-neutralization-of-multiple-strains-of-covid-19-301232221.html
SOURCE Biohaven Pharmaceutical Holding Company Ltd.