Biohaven Enrolls Phase 1a/1b Clinical Trial of BHV-1100, Lead Asset from its ARM™ (Antibody Recruiting Molecule) Platform, in Combination with NK Cell Therapy for the Treatment of Multiple Myeloma
Biohaven Pharmaceutical (NYSE: BHVN) has commenced a Phase 1a/1b clinical trial for BHV-1100, targeting multiple myeloma in patients with minimal residual disease (MRD+). The trial aims to evaluate the safety and efficacy of BHV-1100 combined with autologous cytokine induced memory-like (CIML) natural killer (NK) cells. Enrollment is set for 25 patients prior to autologous stem cell transplant (ASCT). The ARM platform driving BHV-1100 offers potential advantages over traditional therapies, with expectations of significant tumor burden reduction in CD38-positive myeloma cases.
- Initiation of Phase 1a/1b trial for BHV-1100 to treat multiple myeloma, indicating progress in drug development.
- The trial targets newly diagnosed patients with minimal residual disease (MRD+), presenting a focused treatment opportunity.
- BHV-1100 utilizes an innovative ARM platform, enhancing the potential for effective tumor targeting.
- None.
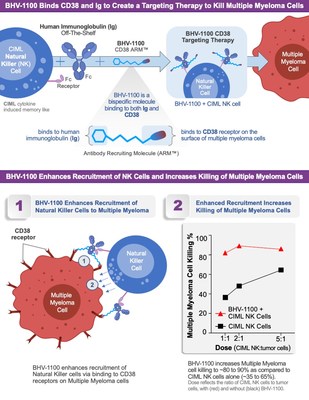
NEW HAVEN, Conn., Oct. 27, 2021 /PRNewswire/ -- Biohaven Pharmaceutical Holding Company Ltd. (NYSE: BHVN), announced the enrollment of the first patient in a Phase 1a/1b trial in Multiple Myeloma using the ARM, BHV-1100, in combination with autologous cytokine induced memory-like (CIML) natural killer (NK) cells and immunoglobulin (Ig) to target and kill multiple myeloma cells expressing the cell surface protein CD38 (Figure 1). BHV-1100 is the lead clinical asset from Biohaven's ARM Platform, developed from a strategic alliance with PeptiDream Inc. This clinical trial will assess the safety and tolerability, as well as exploratory efficacy endpoints, in newly diagnosed multiple myeloma patients who have tested positive for minimal residual disease (MRD+) in first remission prior to autologous stem cell transplant (ASCT).
NK cells are part of the innate immune system, which is designed to recognize and destroy "non-self" or diseased cells in the body. However, tumor cells can evade detection by immune effector cells, allowing the tumor to advance. BHV-1100 targets a cell-surface protein, CD38, that is heavily overexpressed on multiple myeloma and binds to it, recruiting primed autologous cytokine induced memory-like (CIML) natural killer (NK) cells to destroy the tumor.
Charlie Conway, Ph.D., Chief Scientific Officer at Biohaven commented, "While many recent advances have been made to benefit multiple myeloma patients, most patients will unfortunately still relapse. We are excited to investigate BHV-1100 for its ability to recruit autologous CIML NK cells to the site of the tumor. Based on preclinical data from Biohaven Labs, we anticipate that our CD38 targeting ARM-enabled NK cells will kill CD38-positive multiple myeloma cells, and recruit other immune effector cells to assist in reducing the tumor burden."
Biohaven has initiated enrollment in the clinical trial and plans to enroll 25 patients for this single-center, open-label study (ClinicalTrials.gov Identifier: NCT04634435; https://clinicaltrials.gov/ct2/show/NCT04634435). The study will enroll newly diagnosed multiple myeloma patients who have minimal residual disease (MRD+) in first remission prior to an autologous stem cell transplant (ASCT).
David Spiegel M.D., PhD, inventor of the ARM technology and Professor of Chemistry and Pharmacology at Yale University, commented, "This is an important milestone in the development of the ARM therapeutic platform – taking a novel technology from 'benchtop to bedside'. It also highlights Biohaven's commitment to benefit patients in need."
About ARMs: Antibody Recruiting Molecules
ARMs, antibody recruiting molecules, are engineered with modular components that are readily interchangeable, giving the platform tremendous flexibility and rapid development timelines. ARM compounds are being developed at Biohaven Labs to redirect a patient's own antibodies for therapeutic effect with multiple benefits over traditional monoclonal antibody therapies, including the potential for oral dosing. For BHV-1100, the ARM platform is being used to provide antigen targeting to NK cell-based therapies without genetic engineering. This NK cell targeting approach is also being investigated with allogeneic, or 'off-the-shelf', immune cell-based therapies.
About Multiple Myeloma
Multiple myeloma is a type of blood cancer of the plasma cell that develops in the bone marrow, the soft tissue inside our bones. Healthy plasma cells produce antibodies, which are critical for the immune system's ability to recognize disease-causing entities, such as bacteria, viruses and tumor cells. In multiple myeloma, however, genetic abnormalities in a single plasma cell cause it to divide uncontrollably. This leads to the over-production of a single (monoclonal) antibody protein, referred to as an "M protein". Also, these cancerous cells divide to the point of crowding out normal, healthy cells that reside in the bone marrow. Many patients are diagnosed due to symptoms such as bone pain or fractures, kidney failure (thirst, dehydration, confusion), nerve pain, fever, and weakness. The American Cancer Society estimates that approximately 34,920 new cases will be diagnosed, and 12,410 deaths will occur in 2021 from multiple myeloma.
About Biohaven
Biohaven is a commercial-stage biopharmaceutical company with a portfolio of innovative, best-in-class therapies to improve the lives of patients with debilitating neurological and neuropsychiatric diseases, including rare disorders and areas of unmet need. Biohaven's neuro-innovation portfolio includes FDA-approved NURTEC® ODT (rimegepant) for the acute and preventive treatment of migraine and a broad pipeline of late-stage product candidates across three distinct mechanistic platforms: CGRP receptor antagonism for the acute and preventive treatment of migraine; glutamate modulation for obsessive-compulsive disorder, Alzheimer's disease, and spinocerebellar ataxia; and MPO inhibition for amyotrophic lateral sclerosis. More information about Biohaven is available at www.biohavenpharma.com.
About PeptiDream
PeptiDream Inc. is a public (Tokyo Stock Exchange 1st Section 4587) biopharmaceutical company founded in 2006 employing their proprietary Peptide Discovery Platform System (PDPS), a state-of-the-art highly versatile discovery platform which enables the production of highly diverse (trillions) non-standard peptide libraries with high efficiency, for the identification of highly potent and selective hit candidates, which then can be developed into peptide-based, small molecule-based, or peptide-drug-conjugate-based therapeutics. PeptiDream aspires to be a world leader in drug discovery and development to address unmet medical needs and improve the quality of life of patients worldwide. Further information regarding PeptiDream can be found at: www.peptidream.com.
Forward-looking Statement
This news release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements involve substantial risks and uncertainties, including statements that are based on the current expectations and assumptions of Biohaven's management about BHV-1100 as a treatment for multiple myeloma. Forward-looking statements include those related to: Biohaven's ability to effectively develop and commercialize BHV-1100, delays or problems in the supply or manufacture of BHV-1100, complying with applicable U.S. regulatory requirements, the expected timing, commencement and outcomes of Biohaven's planned and ongoing clinical trials, the timing of planned interactions and filings with the FDA, the timing and outcome of expected regulatory filings, the potential commercialization of Biohaven's product candidates, the potential for Biohaven's product candidates to be first in class or best in class therapies and the effectiveness and safety of Biohaven's product candidates. Various important factors could cause actual results or events to differ materially from those that may be expressed or implied by our forward-looking statements. Additional important factors to be considered in connection with forward-looking statements are described in the "Risk Factors" section of Biohaven's Annual Report on Form 10-K for the year ended December 31, 2020, filed with the Securities and Exchange Commission on March 1, 2021, and Biohaven's subsequent filings with the Securities and Exchange Commission. The forward-looking statements are made as of this date and Biohaven does not undertake any obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
NURTEC and NURTEC ODT are registered trademarks of Biohaven Pharmaceutical Ireland DAC.
Neuroinnovation is a trademark of Biohaven Pharmaceutical Holding Company Ltd.
ARM is a trademark of Kleo Pharmaceuticals, Inc.
Biohaven Contact
Dr. Vlad Coric
Chief Executive Officer
Vlad.Coric@biohavenpharma.com
Media Contact
Mike Beyer
Sam Brown Inc.
mikebeyer@sambrown.com
312-961-2502
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/biohaven-enrolls-phase-1a1b-clinical-trial-of-bhv-1100-lead-asset-from-its-arm-antibody-recruiting-molecule-platform-in-combination-with-nk-cell-therapy-for-the-treatment-of-multiple-myeloma-301409304.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/biohaven-enrolls-phase-1a1b-clinical-trial-of-bhv-1100-lead-asset-from-its-arm-antibody-recruiting-molecule-platform-in-combination-with-nk-cell-therapy-for-the-treatment-of-multiple-myeloma-301409304.html
SOURCE Biohaven Pharmaceutical Holding Company Ltd.
FAQ
What is the purpose of the BHV-1100 Phase 1a/1b trial?
How many patients will be enrolled in the BHV-1100 clinical trial?
What is the role of the ARM platform in the BHV-1100 trial?
What patient population is targeted in the BHV-1100 trial?