Biohaven Achieves Positive Topline Results in Pivotal Study of Troriluzole in Spinocerebellar Ataxia (SCA)
Biohaven (NYSE: BHVN) announced positive topline results from a pivotal study of troriluzole in Spinocerebellar Ataxia (SCA). The study met its primary endpoint, showing significant improvement in the modified functional Scale for the Assessment and Rating of Ataxia (f-SARA) after 3 years of treatment. Key findings include:
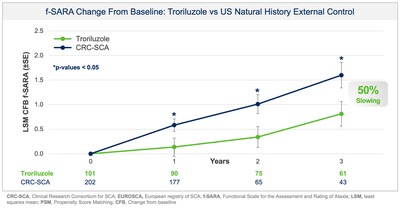
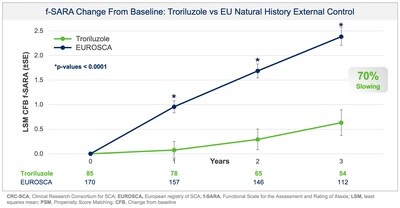
- 50-70% slowing of disease progression, representing a 1.5-2.2 year delay over 3 years
- Statistically significant superiority at 1, 2, and 3 years
- Achieved significance on 9 consecutive, prespecified primary and secondary endpoints
Biohaven plans to submit a New Drug Application (NDA) to the FDA in Q4 2024, with potential for priority review due to orphan drug and fast-track designations. If approved, Biohaven aims to commercialize troriluzole for SCA in the US by 2025.
Biohaven (NYSE: BHVN) ha annunciato risultati positivi di alto livello da uno studio fondamentale su troriluzole per la Atassia Spinocerebellare (SCA). Lo studio ha raggiunto il suo obiettivo primario, mostrando un miglioramento significativo nella Scala Funzionale Modificata per la Valutazione e la Classificazione dell'Atassia (f-SARA) dopo 3 anni di trattamento. I risultati chiave includono:
- 50-70% di rallentamento della progressione della malattia, corrispondente a un ritardo di 1,5-2,2 anni su un periodo di 3 anni
- Superiorità statisticamente significativa a 1, 2 e 3 anni
- Raggiunta significatività su 9 endpoint primari e secondari consecutivi e predefiniti
Biohaven prevede di presentare una Domanda di Nuovo Farmaco (NDA) alla FDA nel Q4 2024, con il potenziale per una revisione prioritaria grazie alle designazioni di farmaco orfano e di via veloce. Se approvato, Biohaven mira a commercializzare il troriluzole per la SCA negli Stati Uniti entro il 2025.
Biohaven (NYSE: BHVN) anunció resultados positivos preliminares de un estudio clave sobre troriluzole en Ataxia Espinocerebelosa (SCA). El estudio alcanzó su objetivo principal, mostrando una mejora significativa en la Escala Funcional Modificada para la Evaluación y Calificación de Ataxia (f-SARA) después de 3 años de tratamiento. Los hallazgos clave incluyen:
- 50-70% de desaceleración de la progresión de la enfermedad, lo que representa un retraso de 1.5-2.2 años durante 3 años
- Superioridad estadísticamente significativa a 1, 2 y 3 años
- Se alcanzó significatividad en 9 puntos finales primarios y secundarios consecutivos y predefinidos
Biohaven planea presentar una Solicitud de Nuevo Medicamento (NDA) a la FDA en el cuarto trimestre de 2024, con potencial para revisión prioritaria debido a designaciones de medicamento huérfano y de vía rápida. Si se aprueba, Biohaven tiene como objetivo comercializar el troriluzole para SCA en EE. UU. para 2025.
Biohaven (NYSE: BHVN)은 Spinocerebellar Ataxia (SCA)에 대한 troriluzole의 중요한 연구에서 긍정적인 최종 결과를 발표했습니다. 이 연구는 주요 목표를 달성했으며, 치료 후 3년 동안 성인용 비정상 운동 평가 척도(f-SARA)에서 상당한 개선을 보여주었습니다. 주요 발견은 다음과 같습니다:
- 질병 진행 속도의 50-70% 느려짐, 이는 3년 동안 1.5-2.2년의 지연을 의미합니다
- 1, 2, 3년 동안 통계적으로 유의미한 우수성
- 9개의 연속적인 사전 지정된 주요 및 보조 지점에서 유의미성을 달성했습니다
Biohaven은 2024년 4분기에 FDA에 신약 신청(NDA)을 제출할 계획이며, 고아약 및 신속 심사 지정으로 우선 심사 가능성이 있습니다. 승인이 될 경우, Biohaven은 2025년까지 미국에서 SCA를 위한 troriluzole을 상용화하는 것을 목표로 하고 있습니다.
Biohaven (NYSE: BHVN) a annoncé des résultats positifs préliminaires d'une étude pivot sur troriluzole dans le cadre de l'Ataxie Spinocérébelleuse (SCA). L'étude a atteint son objectif principal, montrant une amélioration significative dans la Échelle Fonctionnelle Modifiée pour l'Évaluation et la Notation de l'Ataxie (f-SARA) après 3 ans de traitement. Les résultats clés comprennent :
- Ralentissement de la progression de la maladie de 50 à 70 %, représentant un retard de 1,5 à 2,2 ans sur 3 ans
- Supériorité statistiquement significative à 1, 2 et 3 ans
- Atteint de la signification sur 9 points de cheminement primaires et secondaires consécutifs et prédéfinis
Biohaven prévoit de soumettre une Demande de Nouveau Médicament (NDA) à la FDA au 4ème trimestre 2024, avec un potentiel de révision prioritaire en raison des désignations de médicaments orphelins et de voies rapides. Si approuvé, Biohaven vise à commercialiser le troriluzole pour la SCA aux États-Unis d'ici 2025.
Biohaven (NYSE: BHVN) gab positive Zwischenergebnisse aus einer entscheidenden Studie zu troriluzole bei Spinocerebellärer Ataxie (SCA) bekannt. Die Studie erreichte das primäre Ziel und zeigte eine signifikante Verbesserung der modifizierten funktionalen Skala zur Bewertung und Beurteilung von Ataxie (f-SARA) nach 3 Jahren Behandlung. Zu den wichtigsten Ergebnissen gehören:
- Eine Verlangsamung des Krankheitsverlaufs um 50-70%, was eine Verzögerung von 1,5-2,2 Jahren über 3 Jahre darstellt
- Statistisch signifikante Überlegenheit nach 1, 2 und 3 Jahren
- Erreichte Signifikanz bei 9 aufeinanderfolgenden, vorab festgelegten primären und sekundären Endpunkten
Biohaven plant, im 4. Quartal 2024 einen Neue-Arzneimittel-Antrag (NDA) bei der FDA einzureichen, mit der Möglichkeit einer vorrangigen Überprüfung aufgrund von Orphan-Drug- und Fast-Track-Status. Im Falle einer Genehmigung strebt Biohaven an, troriluzole bis 2025 in den USA für SCA zu kommerzialisieren.
- Troriluzole met the primary endpoint in the pivotal SCA study, showing significant improvement in f-SARA scores after 3 years
- Treatment with troriluzole resulted in 50-70% slowing of disease progression, equivalent to 1.5-2.2 years delay over 3 years
- Statistically significant superiority was achieved on 9 consecutive, prespecified primary and secondary endpoints
- Biohaven plans to submit an NDA to the FDA in Q4 2024, with potential for priority review
- The company aims to commercialize troriluzole for SCA in the US by 2025 if approved
- None.
Insights
Analyzing...
- Troriluzole 200 mg dosed orally, once daily, in patients with SCA met the study's primary endpoint on the change from baseline in the modified functional Scale for the Assessment and Rating of Ataxia (f-SARA) at 3 years in all study population genotypes.
- Troriluzole also showed statistically significant superiority after both 1 and 2 years of treatment.
- Troriluzole achieved statistically significant superiority on 9 consecutive, prespecified primary and secondary endpoints.
- SCA patients treated with troriluzole showed a 50
-70% slowing of disease progression, representing 1.5-2.2 years delay in disease progression over the 3-year study period. - Biohaven plans to submit a New Drug Application (NDA) to the US Food and Drug Administration (FDA) for troriluzole in the treatment of all SCA genotypes in 4Q 2024. The application is eligible for a priority review given orphan drug and fast-track designations previously granted by FDA.
- Conference call and webcast to be held today at 8:30am ET
Collectively, data across multiple analyses demonstrate a robust and clinically meaningful slowing of disease progression in SCA patients. These treatment benefits translate into a 50
Dr. Susan Perlman, Director of Ataxia Clinic and Neurogenetics Clinical Trials at the David Geffen School of Medicine at UCLA stated, "SCA is a debilitating, relentlessly progressive disease that destroys quality of life, leaving patients unable to care for themselves, walk, or speak. Troriluzole is the very first treatment to show a delay in disease progression that can give patients additional years of independence, where they can walk without assistance, continue to work, play with their children, and participate in daily activities. This is an exciting and hopeful moment for the SCA community."
Study BHV4157-206-RWE was designed, in discussion with the US Food and Drug Administration (FDA), to assess the effectiveness of troriluzole in SCA after 3 years of treatment as measured by the change from baseline in the f-SARA. The study utilized Phase 3 data and an external control of matched, untreated SCA subjects from the US Clinical Research Consortium for the Study of Cerebellar Ataxia (CRC-SCA) in accordance with FDA's Guidance on Real-World Evidence (RWE) of effectiveness. All endpoints were prespecified, and both the study protocol and statistical analysis plan were submitted to, and reviewed by, FDA prior to topline data analysis. The new analysis doubled the previously available 3 year data with 63 subjects now completing 3 years of treatment with troriluzole and matched to the external control arm. Propensity Score Matching (PSM) was used to ensure that untreated patients from the CRC-SCA study were rigorously matched to treated patients from Study BHV4157-206 on baseline characteristics. The primary objective was to examine the treatment effects of troriluzole for up to 3 years, by comparing data on the f-SARA from patients treated with troriluzole in Study BHV4157-206 to untreated patients from the natural history study. Troriluzole-treated patients demonstrated statistically significant and sustained benefits at years 1, 2 and 3 on the f-SARA compared to a rigorously matched natural history control.
Additionally, prespecified analyses in the protocol employed a separate, independent natural history control from the European SCA natural history study (EUROSCA) for global regulatory purposes. Results using the EUROSCA patients, in addition to a pooled analysis using both CRC-SCA and EUROSCA patients, as the external controls were also statistically significant and consistent with the primary efficacy analysis at all timepoints (see Figure 2 and Figure 3). The addition of EUROSCA data increased the external control sample size and added to the robustness of the statistically significant treatment differences at years 1, 2, and 3, favoring troriluzole.
Jeremy Schmahmann, M.D., Professor of Neurology at Harvard Medical School and Founding Director of the Ataxia Center at Massachusetts General Hospital commented, "The stabilization of SCA symptoms as reflected by the topline data at 3 years along with the previously reported reductions in falls show the therapeutic potential of troriluzole. I cannot underscore enough the impact of a potential treatment that can slow SCA disease progression and the effect on patients and caregivers who have helplessly watched generations of family members deteriorate and die from SCA. These new data provide support for troriluzole as a safe and effective once daily treatment for patients with SCA."
Spinocerebellar ataxia is a group of dominantly inherited neurodegenerative disorders characterized by progressive loss of voluntary motor control and atrophy of the cerebellum, brainstem and spinal cord. Patients experience significant morbidity, including progression to a wheelchair, impaired gait leading to falls, inability to communicate due to speech impairment, difficulty swallowing, and premature death. While signs and symptoms can appear anytime from childhood to late adulthood, SCA typically presents in early adulthood and progresses over a number of years. Currently, there are no FDA-approved treatments and no cure for SCA.
Vlad Coric, M.D., Chief Executive Officer and Chairman of Biohaven stated, "Advancing new therapies for patients with rare diseases is often a multiyear process of collaboration across academic, patient advocacy, regulatory and industry partners. The Biohaven team has always been committed to rigorously following the science in this area, and through our partnership with the National Ataxia Foundation and collaboration with leading SCA experts across the globe, our SCA development program has provided the first evidence of a clinically meaningful treatment benefit as well as slowing disease progression in SCA patients. We were excited to receive the positive topline results from Study BHV4157-206-RWE, which was designed with FDA input and pursuant to the principles outlined in the FDA's guidance for the use of real-world evidence. The need for treatments for this deadly neurodegenerative disease is urgent. As a company, we remain committed to developing novel therapies for patients living with rare disorders with no approved therapies, like SCA. We look forward to interacting with regulatory agencies to bring troriluzole to patients with SCA."
Andrew Rosen, Chief Executive Officer of the National Ataxia Foundation (NAF), shared, "Biohaven was the first company to join NAF's Drug Development Collaborative (DDC), a group of pharmaceutical companies dedicated to bringing together advocates, clinicians, regulatory agencies, and the patient community to advance research and facilitate the development of therapies for ataxia. Today's topline results are the culmination of years dedicated to studying troriluzole as a treatment for SCA. Patients and families have been waiting for decades for a treatment that could slow disease progression in this devastating and relentlessly progressive disorder".
Based upon the topline data from Study BHV4157-206-RWE, and previous safety and efficacy data from the troriluzole development program in SCA, Biohaven plans to submit a New Drug Application (NDA) to the FDA in Q4 2024. The troriluzole development program has generated the largest clinical trial dataset in SCA and now has follow-up in some patients treated with troriluzole for over 5 years. Biohaven has previously received both Fast-Track and Orphan drug designation (ODD) from the FDA, and ODD from the European Medicines Agency, for troriluzole in SCA. An NDA with ODD is eligible for priority FDA review. Biohaven will be prepared to commercialize SCA in the US in 2025, if ultimately approved, based on potential priority review timelines.
Conference Call and Webcast Details
Biohaven will hold a live conference call and webcast today at 8:30 a.m. Eastern Time. The webcast may be accessed via the Investor Relations portion of Biohaven's website at https://ir.biohaven.com/events-presentations/events. To participate in the live conference call via telephone, please register here. Upon registering, a dial-in number and unique PIN will be provided to join the conference call.
About Troriluzole
Troriluzole is a new chemical entity (NCE) and third-generation novel prodrug that modulates glutamate, the most abundant excitatory neurotransmitter in the human body. The primary mode of action of troriluzole is reducing synaptic levels of glutamate. Troriluzole increases glutamate uptake from the synapse, by augmenting the expression and function of excitatory amino acid transporters located on glial cells that play a key role in clearing glutamate from the synapse. Troriluzole has the potential to be developed in a number of other diseases associated with excessive glutamate. More information about troriluzole can be found at the Company's website: https://www.biohaven.com/pipeline/clinical-programs/glutamate/.
About Biohaven
Biohaven is a biopharmaceutical company focused on the discovery, development, and commercialization of life-changing treatments in key therapeutic areas, including immunology, neuroscience, and oncology. The company is advancing its innovative portfolio of therapeutics, leveraging its proven drug development experience and multiple proprietary drug development platforms. Biohaven's extensive clinical and preclinical programs include Kv7 ion channel modulation for epilepsy and mood disorders; extracellular protein degradation for immunological diseases; TRPM3 antagonism for migraine and neuropathic pain; TYK2/JAK1 inhibition for neuroinflammatory disorders; glutamate modulation for OCD and SCA (spinocerebellar ataxia); myostatin inhibition for neuromuscular and metabolic diseases, including SMA and obesity; antibody recruiting bispecific molecules and antibody drug conjugates for cancer. For more information, visit www.biohaven.com.
Forward-looking Statements
This news release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including statements about Biohaven Ltd. and our planned and ongoing clinical trials, the timing of and the availability of data from those trials, the timing and our decisions to proceed with our planned regulatory filings (including our plans to submit a NDA to the FDA for troriluzole in the treatment of all SCA genotypes in 4Q 2024), the timing of and our ability to obtain regulatory approvals for our product candidates (including the timing of the regulatory approval for troriluzole in order to commercialize SCA in
Investor Contact:
Jennifer Porcelli
Vice President, Investor Relations
jennifer.porcelli@biohavenpharma.com
+1 (201) 248-0741
Media Contact:
Mike Beyer
Sam Brown Inc.
mikebeyer@sambrown.com
+1 (312) 961-2502
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/biohaven-achieves-positive-topline-results-in-pivotal-study-of-troriluzole-in-spinocerebellar-ataxia-sca-302255056.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/biohaven-achieves-positive-topline-results-in-pivotal-study-of-troriluzole-in-spinocerebellar-ataxia-sca-302255056.html
SOURCE Biohaven Ltd.