BD Announces 510(k) Clearance of Expanded Indications for the Rotarex™ Atherectomy System
BD (Becton, Dickinson and Company) has received 510(k) clearance from the FDA for the Rotarex™ Atherectomy System, expanding its indications to include treatment in peripheral arteries with stents, stent grafts, and bypasses. This device can now assist in the treatment of in-stent restenosis, a complication often arising in patients with peripheral arterial disease (PAD). With over a decade of clinical use, the Rotarex™ System is recognized for its efficacy in effectively managing arterial lesions and improving patient outcomes.
- FDA clearance for expanded use of the Rotarex™ Atherectomy System.
- New indications allow treatment within stented and bypass vessels.
- Proven efficacy over more than 10 years of clinical usage.
- None.
FRANKLIN LAKES, N.J., Oct. 6, 2021 /PRNewswire/ -- BD (Becton, Dickinson and Company) (NYSE: BDX), a leading global medical technology company, today announced it has received 510(k) clearance for expanded indications from the U.S. Food and Drug Administration (FDA) for the Rotarex™ Atherectomy System.
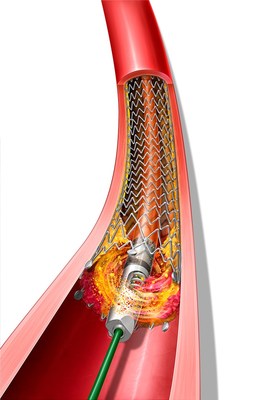
The Rotarex™ Atherectomy System is a rotational excisional device that is built to remove and aspirate varying lesion morphologies including plaque and thrombus in the peripheral arteries. The Rotarex™ System, already cleared for use in native arterial vessels, now has the expanded indications to treat within peripheral arteries fitted with stents, stent grafts, and native or artificial bypasses.
"The Rotarex™ Atherectomy System is quick and efficient for treating arterial lesions," said Dr. Prakash Krishnan, a cardiologist from New York. "With ability to use it for both atherectomy and thrombectomy, it is now a great product to be able to treat in-stent restenosis. Rotarex has been a great device for me to have in my practice, and I am excited about these new indications."
The FDA clearance of the Rotarex™ Atherectomy System's new expanded indications follows more than 10 years of real-world clinical experience involving the treatment of thousands of patients globally. Now, physicians can use a proven tool to address some of their most challenging peripheral arterial disease (PAD) lesions, including the treatment of in-stent restenosis.
"I have had the ability to use the Rotarex™ Atherectomy System for over 10 years both within and outside the U.S.," said Dr. Miguel Montero-Baker, a vascular surgeon from Houston. "This indication expansion is exciting and will allow me to utilize a device I trust to care for my complex PAD patients."
PAD is a potentially debilitating disease that can lead to increased risk of cardiovascular complications and limb amputation. A healthy diet, exercise and cessation of smoking can help mitigate the development of PAD, which includes the formation of atherosclerosis and thrombus (blood clots) in arteries in the legs. However, treatment for PAD may be necessary if the arteries are blocked. Atherectomy and thrombectomy are minimally invasive techniques that use a catheter to remove the plaque and blood clot buildup, respectively, to increase blood flow through the diseased areas. The unique mechanism of action of Rotarex™ Atherectomy Device has enabled the simultaneous treatment of both plaque and thrombus.
"The additional indications provide physicians a one-of-a-kind, ideal solution for treating complex lesions associated with peripheral arterial disease and further demonstrates our responsibility as an industry to develop innovative technologies to help with the fight against PAD," said Paddy O'Brien, worldwide president of Peripheral Intervention for BD.
About BD
BD is one of the largest global medical technology companies in the world and is advancing the world of health by improving medical discovery, diagnostics and the delivery of care. The company supports the heroes on the frontlines of health care by developing innovative technology, services and solutions that help advance both clinical therapy for patients and clinical process for health care providers. BD and its 70,000 employees have a passion and commitment to help enhance the safety and efficiency of clinicians' care delivery process, enable laboratory scientists to accurately detect disease and advance researchers' capabilities to develop the next generation of diagnostics and therapeutics. BD has a presence in virtually every country and partners with organizations around the world to address some of the most challenging global health issues. By working in close collaboration with customers, BD can help enhance outcomes, lower costs, increase efficiencies, improve safety and expand access to health care. For more information on BD, please visit bd.com or connect with us on LinkedIn at www.linkedin.com/company/bd1/ and Twitter @BDandCo.
Contacts: | |
Media: | Investors: |
Troy Kirkpatrick | Nadia Goncalves |
BD Public Relations | BD Investor Relations |
858.617.2361 | 201.847.5934 |
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/bd-announces-510k-clearance-of-expanded-indications-for-the-rotarex-atherectomy-system-301393474.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/bd-announces-510k-clearance-of-expanded-indications-for-the-rotarex-atherectomy-system-301393474.html
SOURCE BD (Becton, Dickinson and Company)
FAQ
What new clearance did BDX receive for the Rotarex™ Atherectomy System?
When was the FDA clearance for the Rotarex™ System announced?
How does the Rotarex™ Atherectomy System benefit patients with PAD?