New Survey Finds Women Are Skipping Their OB/GYN Exams, Increasing Risks for Cervical Cancer
Rhea-AI Summary
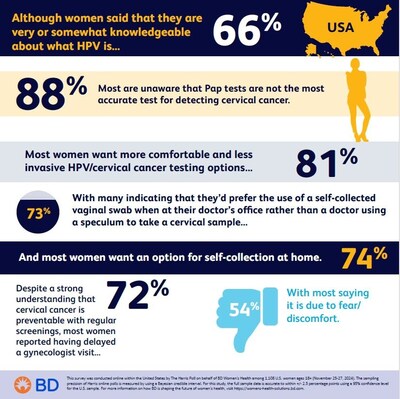
BD (NYSE: BDX) has released a concerning survey about women's gynecological health practices. The Harris Poll survey, conducted in November 2024 among 1,100+ U.S. women, revealed that 72% have delayed gynecology visits, with 54% citing fear or discomfort and 49% mentioning scheduling challenges.
Key findings include: 50% of women are uncertain about cervical cancer screening frequency; 81% desire more comfortable testing options; and 73% show interest in self-collection methods at doctor's offices. Despite 66% claiming knowledge about HPV, 88% are unaware that Pap tests aren't the most accurate for detecting cervical cancer.
The company's BD Onclarity™ HPV Assay, FDA-approved for self-collection, identifies more high-risk HPV types than other tests. Major health organizations, including WHO and ACS, now recommend self-collection for HPV screening. WHO aims to eliminate cervical cancer as a public health issue by 2030, noting it's currently the fourth most common cancer in women worldwide.
Positive
- None.
Negative
- None.
News Market Reaction – BDX
On the day this news was published, BDX declined 0.32%, reflecting a mild negative market reaction.
Data tracked by StockTitan Argus on the day of publication.
Women Are Seeking More Convenience and Less Discomfort in Testing Options
FRANKLIN LAKES, N.J., Jan. 9, 2025 /PRNewswire/ -- BD (Becton, Dickinson and Company) (NYSE: BDX), a leading global medical technology company, today announced the results of a new survey, revealing that
According to the survey, conducted online by The Harris Poll among over 1,100 adult women in the
"The fact that women are skipping this potentially life-saving screening due to fear or scheduling makes it clear that the health industry needs to make the process more comfortable and convenient," said Dr. Jeff Andrews, a board-certified OB/GYN physician and vice president, Medical Affairs at BD. "Self-collection of vaginal samples reduces both the discomfort and time associated with a pelvic exam and is a critical step forward in cervical cancer screening."
Desire for Better Testing Options
The new study found that
The survey found that while
"Medical research continues to be focused on the worthy goal of finding 'a cure for cancer,' but we're already able to help prevent cervical cancer today," said Nikos Pavlidis, worldwide president of BD Diagnostic Solutions. "The combination of vaccines, more precise HPV tests and self-collection will be important factors as we work to eliminate cervical cancer as a public health risk."
The BD Onclarity™ HPV Assay is FDA-approved for self-collection, and it also identifies more individual high-risk types or strains of HPV than any other test. Being able to identify more individual types of HPV means that clinicians can track those types across a patient's visits to more effectively manage high-risk cases and better guide follow-up for low-risk patients. This targeted approach helps ensure that women and people with a cervix receive the most appropriate care for their situation and avoid return visits to the doctor's office for invasive tests that may not be necessary.
Guidelines from a growing number of national and international agencies, including the World Health Organization (WHO), the American Cancer Society (ACS), the American Society for Coloscopy and Cervical Pathology (ASCCP) and the
According to the WHO, a woman dies of cervical cancer every two minutes. Cervical cancer is the fourth most common cancer in women worldwide, both in terms of incidence and deaths, but it is preventable with regular screening. The WHO aims to eliminate cervical cancer as a public health issue by 2030.
This survey was conducted online within
For the complete research method and additional survey results, please contact mela.sera@bd.com.
About BD
BD is one of the largest global medical technology companies in the world and is advancing the world of health by improving medical discovery, diagnostics and the delivery of care. The company supports the heroes on the frontlines of health care by developing innovative technology, services and solutions that help advance both clinical therapy for patients and clinical process for health care providers. BD and its more than 70,000 employees have a passion and commitment to help enhance the safety and efficiency of clinicians' care delivery process, enable laboratory scientists to accurately detect disease and advance researchers' capabilities to develop the next generation of diagnostics and therapeutics. BD has a presence in virtually every country and partners with organizations around the world to address some of the most challenging global health issues. By working in close collaboration with customers, BD can help enhance outcomes, lower costs, increase efficiencies, improve safety and expand access to health care. For more information on BD, please visit bd.com or connect with us on LinkedIn at www.linkedin.com/company/bd1/, X (formerly Twitter) @BDandCo or Instagram @becton_dickinson
Contacts: | |
Media | Investors |
Mela Sera, APR | Adam Reiffe Sr. Director, Investor Relations 201.847.6927 |
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/new-survey-finds-women-are-skipping-their-obgyn-exams-increasing-risks-for-cervical-cancer-302346535.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/new-survey-finds-women-are-skipping-their-obgyn-exams-increasing-risks-for-cervical-cancer-302346535.html
SOURCE BD (Becton, Dickinson and Company)