Avicanna Reports Full Year 2024 Audited Financial Statement
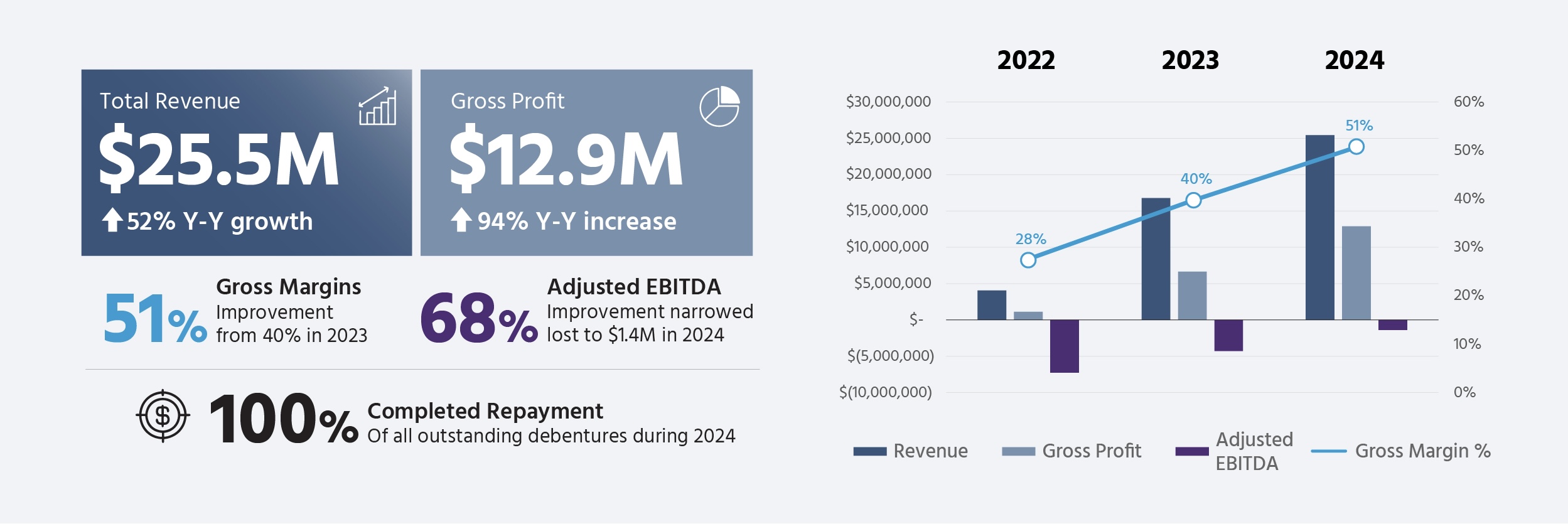
Avicanna (AVCNF) reported strong financial results for 2024, with revenue reaching $25.5 million, marking a 52% increase from 2023. The company achieved significant improvements in profitability, with gross profits rising 94% to $12.9 million and gross margins expanding to 51%.
Key operational highlights include the initiation of a 1,000-patient medical cannabis study, expansion to 42 proprietary commercial SKUs, and completion of several clinical studies, including research on epidermolysis bullosa and musculoskeletal pain. The company's adjusted EBITDA loss narrowed by 68% to $1.4 million, and they successfully repaid $1.3 million in debentures.
Notable achievements include obtaining drug registration in Colombia for Trunerox™, delivering proprietary topical products to a multinational pharmaceutical company, and securing two new US patents for their cannabinoid delivery technologies.
Avicanna (AVCNF) ha riportato risultati finanziari solidi per il 2024, con ricavi che hanno raggiunto 25,5 milioni di dollari, segnando un incremento del 52% rispetto al 2023. L'azienda ha ottenuto miglioramenti significativi nella redditività, con i profitti lordi che sono aumentati del 94% a 12,9 milioni di dollari e i margini lordi che si sono espansi al 51%.
I principali punti operativi includono l'avvio di uno studio clinico sulla cannabis medica con 1.000 pazienti, l'espansione a 42 SKU commerciali proprietari e il completamento di diversi studi clinici, tra cui ricerche sulla epidermolisi bollosa e sul dolore muscoloscheletrico. La perdita di EBITDA rettificato dell'azienda si è ridotta del 68% a 1,4 milioni di dollari e hanno rimborsato con successo 1,3 milioni di dollari in obbligazioni.
Tra i risultati notevoli ci sono l'ottenimento della registrazione del farmaco in Colombia per Trunerox™, la fornitura di prodotti topici proprietari a una multinazionale farmaceutica e l'acquisizione di due nuovi brevetti statunitensi per le loro tecnologie di somministrazione dei cannabinoidi.
Avicanna (AVCNF) reportó resultados financieros sólidos para 2024, con ingresos alcanzando 25.5 millones de dólares, marcando un aumento del 52% en comparación con 2023. La compañía logró mejoras significativas en rentabilidad, con ganancias brutas aumentando un 94% a 12.9 millones de dólares y márgenes brutos expandiéndose al 51%.
Los aspectos operativos clave incluyen el inicio de un estudio de cannabis medicinal con 1,000 pacientes, la expansión a 42 SKU comerciales propietarios y la finalización de varios estudios clínicos, incluyendo investigaciones sobre epidermólisis bullosa y dolor musculoesquelético. La pérdida de EBITDA ajustado de la empresa se redujo en un 68% a 1.4 millones de dólares, y lograron pagar 1.3 millones de dólares en bonos.
Logros notables incluyen la obtención de la inscripción de un medicamento en Colombia para Trunerox™, la entrega de productos tópicos propietarios a una compañía farmacéutica multinacional y la obtención de dos nuevas patentes en EE. UU. para sus tecnologías de entrega de cannabinoides.
Avicanna (AVCNF)는 2024년 강력한 재무 결과를 보고했으며, 수익은 2,550만 달러에 도달하여 2023년 대비 52% 증가했습니다. 회사는 수익성에서 중요한 개선을 이루었으며, 총 이익은 94% 증가하여 1,290만 달러에 이르렀고, 총 마진은 51%로 확대되었습니다.
주요 운영 하이라이트에는 1,000명의 환자를 대상으로 하는 의료용 대마초 연구 시작, 42개의 독점 상업 SKU로의 확장, 그리고 피부박리증 및 근골격계 통증에 대한 연구를 포함한 여러 임상 연구의 완료가 포함됩니다. 회사의 조정 EBITDA 손실은 68% 줄어들어 140만 달러가 되었고, 130만 달러의 채권을 성공적으로 상환했습니다.
주목할 만한 성과로는 Trunerox™의 콜롬비아에서의 약물 등록 획득, 다국적 제약회사에 독점적인 국소 제품 제공, 그리고 그들의 카나비노이드 전달 기술에 대한 두 개의 새로운 미국 특허 확보가 있습니다.
Avicanna (AVCNF) a rapporté de solides résultats financiers pour 2024, avec des revenus atteignant 25,5 millions de dollars, marquant une augmentation de 52 % par rapport à 2023. L'entreprise a réalisé des améliorations significatives en matière de rentabilité, avec des bénéfices bruts en hausse de 94 % à 12,9 millions de dollars et des marges brutes s'élevant à 51 %.
Les points opérationnels clés comprennent le lancement d'une étude sur le cannabis médical avec 1 000 patients, l'expansion à 42 SKU commerciaux propriétaires, et l'achèvement de plusieurs études cliniques, y compris des recherches sur l'épidermolyse bulleuse et la douleur musculosquelettique. La perte d'EBITDA ajusté de l'entreprise a diminué de 68 % pour atteindre 1,4 million de dollars, et elle a réussi à rembourser 1,3 million de dollars en obligations.
Parmi les réalisations notables, on trouve l'obtention de l'enregistrement d'un médicament en Colombie pour Trunerox™, la livraison de produits topiques propriétaires à une entreprise pharmaceutique multinationale, et l'obtention de deux nouveaux brevets américains pour leurs technologies de délivrance de cannabinoïdes.
Avicanna (AVCNF) hat starke Finanzergebnisse für 2024 gemeldet, mit einem Umsatz von 25,5 Millionen Dollar, was einem Anstieg von 52% im Vergleich zu 2023 entspricht. Das Unternehmen erzielte erhebliche Verbesserungen bei der Rentabilität, wobei die Bruttogewinne um 94% auf 12,9 Millionen Dollar stiegen und die Bruttomargen auf 51% anstiegen.
Wichtige betriebliche Höhepunkte sind der Beginn einer medizinischen Cannabisstudie mit 1.000 Patienten, die Expansion auf 42 proprietäre kommerzielle SKUs und der Abschluss mehrerer klinischer Studien, einschließlich Forschungen zu epidermolysis bullosa und muskuloskelettalen Schmerzen. Der bereinigte EBITDA-Verlust des Unternehmens verringerte sich um 68% auf 1,4 Millionen Dollar, und sie haben erfolgreich 1,3 Millionen Dollar an Anleihen zurückgezahlt.
Bemerkenswerte Errungenschaften umfassen die Erlangung der Arzneimittelregistrierung in Kolumbien für Trunerox™, die Lieferung proprietärer topischer Produkte an ein multinationales Pharmaunternehmen und die Sicherung von zwei neuen US-Patenten für ihre Cannabinoid-Abgabeteknologienen.
- 52% revenue growth to $25.5M in 2024
- 94% increase in gross profit to $12.9M
- Gross margin improvement from 40% to 51%
- 68% improvement in adjusted EBITDA loss
- Successful debt repayment of $1.3M
- Two new US patents secured for drug delivery technologies
- Drug registration obtained in Colombia for Trunerox™
- Still operating at an EBITDA loss of $1.4M
2024 Revenue of
Consolidated Gross Margins of
TORONTO, April 14, 2025 (GLOBE NEWSWIRE) -- Avicanna Inc. (“Avicanna” or the “Company) (TSX: AVCN) (OTCQX: AVCNF) (FSE: 0NN), a biopharmaceutical company focused on the development, manufacturing, and commercialization of plant-derived cannabinoid-based products, is pleased to announce that its full year 2024 results, audited financial statements with management’s discussion and analysis have been filed.
Management Commentary:
"We are proud to report our most successful year to date, marked by improved financial results and continued advancements in our commercial, R&D and clinical programs. In 2024, we strengthened our financial foundation, achieved self-sufficiency, and established a solid basis for further growth, international expansion, and innovation. We remain committed to our mission of advancing cannabinoid-based medicine and are energized by the prospects that lie ahead as we continue to expand and strengthen our core business pillars " stated Aras Azadian, CEO of Avicanna.
Full Year 2024 Financial Highlights:
- Annual Revenue: Achieved record revenue of
$25.5 million for the year ended December 31, 2024, supported by$6.6 million in revenue for the fourth quarter. This represents a52% year-over-year growth from 2023 and was driven by both Canadian and international business segments. - Gross Profit Growth: Reported a year-over-year gross profit of
$12.9 million , representing an increase of94% compared to 2023. - Gross Margin Improvement: Consolidated Gross margins improved to
51% in 2024, representing an increase of40% in 2023. This improvement in gross margin is attributed to continued optimization efforts and an increased licensing and service revenue. - Adjusted EBITDA: Annual adjusted EBITDA improved by
68% from 2023, narrowing the loss to$1.4 million for 2024, compared to a loss of$4.3 million in 2023. - Debt Repayment: The Company repaid the outstanding principal balance of
$1.3 million on its Non-Convertible Debentures issued in August 2023.
Other 2024 Highlights:
Initiation of Medical Cannabis Real World Evidence Study by MyMedi.ca (“RWE Study”): A prospective, non-interventional, observational study aimed to enroll 1,000 patients across Canada to better understand the potential therapeutic use of medical cannabis and potential impact of medical cannabis on pain, sleep, anxiety, depression, and epilepsy. The RWE Study is led by Dr. Hance Clarke, President of The Canadian Pain Society and the CCIC is leading the RWE Study.
Canadian Commercial Advancements in 2024: The Company completed the year with 42 proprietary commercial SKUs and 136 unique commercial listings. The Company sold approximately 200,000 units during 2024, an increase of
Completion of Study in Patients with Epidermolysis Bullosa at The Hospital for Sick Children Evaluating Wound Healing, Pain, and Itch (“EB Study”): The EB Study led by Elena Pope, MD, M.Sc., FRCPC, Head of Dermatology at The Hospital for Sick Children in Toronto, evaluated the tolerability and efficacy of a proprietary
Completion of Topical Gel Observational Real-World Evidence Study in Patients with Musculoskeletal Pain and Inflammation (“MPI RWE Study”): The MPI RWE Study evaluated patient-reported efficacy of the proprietary Transdermal Gel containing
Avicanna LATAM SAS obtained indication-specific Drug Registration in Colombia with Trunerox™: Trunerox™ was approved in Colombia by the Colombian National Institute of Drug and Food Surveillance (El Instituto Nacional de Vigilancia de Medicamentos y Alimentos – “INVIMA”) as a drug for the treatment of severe seizures related to Lennox-Gastaut Syndrome and Dravet Syndrome. The approval permits Avicanna LATAM SAS to manufacture and commercialize Trunerox™ in Colombia for the approved indications which are two rare epileptic disorders classified as epileptic encephalopathies. Trunerox™ has not been approved as a drug in Canada by Health Canada.
Avicanna Completed the First Delivery of Proprietary Topical Products to a Multinational Pharmaceutical Company in 2024: The products include
United States Patent and Trademark Office (“USPTO”) Issued Patent No. US 12,064,461 B2 and No. US 11,998,632 B2 to Avicanna: US Patent No. US 12,064,461 B2 covers Avicanna’s deep penetrating topical cannabinoid composition and methods for treating musculoskeletal inflammation and pain; and US Patent No. US 11,998,632 B2 covers Avicanna’s “SEDDS” or the self-emulsifying drug delivery system technology for oral cannabinoid composition and methods of treating neuropathic pain.
2024 Earnings Call and Corporate Update:
The Company’s CEO, Mr. Aras Azadian, and CFO, Mr. Phillip Cardella, will host an earnings call and corporate update at 8:30 am ET, Tuesday, April 22, 2025. Interested parties may register by clicking below:
About Avicanna:
Avicanna is a commercial-stage international biopharmaceutical company focused on the advancement and commercialization of cannabinoid-based products and formulations for the global medical and pharmaceutical market segments. Avicanna has an established scientific platform including R&D and clinical development leading to the commercialization of more than thirty proprietary, evidence-based finished products and supporting four commercial stage business pillars.
- Medical Cannabis formulary (RHO Phyto™): The formulary offers a diverse range of proprietary products including oral, sublingual, topical, and transdermal deliveries with varying ratios of cannabinoids, supported by ongoing patient and medical community education. RHO Phyto is an established brand in Canada currently available nationwide across several channels and expanding into new international markets.
- Medical cannabis care platform (MyMedi.ca): MyMedi.ca is a medical cannabis care platform formed with the aim to better serve medical cannabis patients’ needs and enhance the medical cannabis patients’ journey. MyMedi.ca is operated by Northern Green Canada Inc. and features a diverse portfolio of products and bilingual pharmacist-led patient support programs. MyMedi.ca also provides specialty services to distinct patient groups such as veterans and collaborates with public and private payers for adjudication and reimbursement. MyMedi.ca provides educational resources to the medical community to facilitate the incorporation of medical cannabis into health care regimens.
- Pharmaceutical pipeline: Leveraging Avicanna’s scientific platform, vertical integration, and real-world evidence, Avicanna has developed a pipeline of proprietary, indication-specific cannabinoid-based candidates that are in various stages of clinical development. These cannabinoid-based candidates aim to address unmet needs in the areas of dermatology, chronic pain, and various neurological disorders.
- Active pharmaceutical ingredients (Aureus Santa Marta™): Active pharmaceutical ingredients supplied by the Company’s majority owned subsidiary Santa Marta Golden Hemp SAS (“SMGH”) is a commercial-stage business dedicated to providing various forms of high-quality CBD, THC and CBG to the Company’s international partners for use in the development and production of food, cosmetics, medical, and pharmaceutical products. SMGH also forms part of the Company’s supply chain and is a source of reliable input products for its consumer retail, medical cannabis, and pharmaceutical products globally.
SOURCE Avicanna Inc.
Stay Connected
For more information about Avicanna, visit our website or contact Ivana Maric by email at info@avicanna.com.
Cautionary Note Regarding Forward-Looking Information and Statements
This news release contains “forward-looking information” within the meaning of applicable securities laws. Forward-looking information contained in this news release may be identified by the use of words such as, “may”, “would”, “could”, “will”, “likely”, “expect”, “anticipate”, “believe”, “intend”, “plan”, “forecast”, “project”, “estimate”, “outlook” and other similar expressions. Forward-looking information contained in this news release includes, without limitation, statements related to the Offering, the use of proceeds of the Offering, the receipt of all approvals of the Toronto Stock Exchange in connection with the Offering, statements with respect to the Company’s future business operations, the opinions or beliefs of management and future business goals. Although the Company believes that the expectations and assumptions on which such forward looking information is based are reasonable, undue reliance should not be placed on the forward-looking information because the Company can give no assurance that they will prove to be correct. Actual results and developments may differ materially from those contemplated by these statements. Forward-looking information is subject to a variety of risks and uncertainties that could cause actual events or results to differ materially from those projected in the forward-looking information. Such risks and uncertainties include, but are not limited to current and future market conditions, including the market price of the common shares of the Company, and the risk factors set out in the Company’s annual information form dated April 11, 2025, filed with the Canadian securities regulators and available under the Company’s profile on SEDAR+ at www.sedarplus.ca. The statements in this news release are made as of the date of this release. The Company disclaims any intent or obligation to update any forward-looking information, whether as a result of new information, future events or results or otherwise, other than as required by applicable securities laws.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/796929bf-c32f-430f-bebb-74eb10be0a8f








