Asensus Surgical Receives FDA 510(k) Clearance for Senhance Surgical System in Urology
Asensus Surgical (NYSE American: ASXC) has received FDA 510(k) clearance for an expanded indication to treat adult and pediatric Urology patients with the Senhance® Surgical System in the United States. This clearance opens up a significant market, with over 185,000 urological surgical procedures performed annually in the U.S. The Senhance System offers precision and advanced digital capabilities, including 3mm and 5mm instruments and digital integrations, making it well-suited for urological procedures.
The system aims to increase surgeon control and reduce variability through Augmented Intelligence and deep learning capabilities. This expansion builds on the system's successful use in Urology procedures outside the U.S. for several years, now bringing its benefits to the U.S. patient population.
Asensus Surgical (NYSE American: ASXC) ha ricevuto l'
Il sistema mira a aumentare il controllo del chirurgo e ridurre la variabilità attraverso l'Intelligenza Aumentata e le capacità di apprendimento profondo. Questa espansione si basa sull'uso di successo del sistema in procedure urologiche al di fuori degli Stati Uniti per diversi anni, ora portando i suoi benefici alla popolazione paziente statunitense.
Asensus Surgical (NYSE American: ASXC) ha recibido la autorización 510(k) de la FDA para una indicación expandida para tratar a pacientes urológicos adultos y pediátricos con el Sistema Quirúrgico Senhance® en Estados Unidos. Esta autorización abre un mercado significativo, con más de 185,000 procedimientos quirúrgicos urológicos realizados anualmente en EE. UU. El Sistema Senhance ofrece precisión y capacidades digitales avanzadas, incluyendo instrumentos de 3mm y 5mm e integraciones digitales, lo que lo hace muy adecuado para procedimientos urológicos.
El sistema tiene como objetivo aumentar el control del cirujano y reducir la variabilidad a través de la Inteligencia Aumentada y capacidades de aprendizaje profundo. Esta expansión se basa en el uso exitoso del sistema en procedimientos urológicos fuera de los EE. UU. durante varios años, ahora llevando sus beneficios a la población de pacientes estadounidenses.
Asensus Surgical (NYSE American: ASXC)는 미국에서 Senhance® 수술 시스템을 사용하여 성인 및 소아 비뇨기과 환자를 치료하기 위한 확대 적시를 위한 FDA 510(k) 승인을 받았습니다. 이 승인은 연간 185,000건 이상의 비뇨기 수술이 시행되는 중요한 시장을 열어줍니다. Senhance 시스템은 정밀도 및 고급 디지털 기능, 3mm 및 5mm 도구와 디지털 통합을 포함하여 비뇨기과 시술에 매우 적합합니다.
이 시스템은 증강 지능 및 심층 학습 기능을 통해 의사의 통제력을 높이고 변동성을 줄이기 위해 설계되었습니다. 이번 확장은 수년 간 미국 외부에서 비뇨기과 시술에 성공적으로 사용된 시스템의 사용을 기반으로 하며, 이제 그 혜택을 미국 환자들에게 제공합니다.
Asensus Surgical (NYSE American: ASXC) a reçu l'
Le système vise à accroître le contrôle du chirurgien et à réduire la variabilité grâce à l'Intelligence Augmentée et aux capacités d'apprentissage profond. Cette expansion s'appuie sur l'utilisation réussie du système dans des procédures urologiques en dehors des États-Unis pendant plusieurs années, apportant désormais ses bénéfices à la population de patients américains.
Asensus Surgical (NYSE American: ASXC) hat die FDA 510(k) Zulassung für eine erweiterte Indikation zur Behandlung von urologischen Patienten im Erwachsenen- und Kindesalter mit dem Senhance® Chirurgischen System in den Vereinigten Staaten erhalten. Diese Zulassung eröffnet einen bedeutenden Markt, in dem jährlich über 185.000 urologische chirurgische Eingriffe in den USA durchgeführt werden. Das Senhance-System bietet Präzision und fortschrittliche digitale Fähigkeiten, einschließlich Instrumenten mit 3mm und 5mm sowie digitalen Integrationen, was es besonders geeignet für urologische Verfahren macht.
Das System zielt darauf ab, die Kontrolle des Chirurgen zu erhöhen und Variabilität zu reduzieren durch erweiterte Intelligenz und Deep Learning-Fähigkeiten. Diese Erweiterung basiert auf dem erfolgreichen Einsatz des Systems in urologischen Verfahren außerhalb der USA über mehrere Jahre und bringt nun seine Vorteile in die US-Patientenpopulation.
- FDA 510(k) clearance received for expanded indication in Urology
- Access to a market of over 185,000 annual urological procedures in the U.S.
- Senhance System offers precision and advanced digital capabilities suited for urological procedures
- System includes 3mm and 5mm instruments and digital integrations
- Potential to increase surgeon control and reduce variability through AI and deep learning
- None.
Insights
The FDA 510(k) clearance for the Senhance Surgical System's use in Urology is a significant milestone for Asensus Surgical. This clearance opens up a new market within the U.S. for the company's advanced digital surgical tools, which are already being used for Urology procedures abroad. The inclusion of pediatric patients also expands the potential user base. The system's augmented intelligence and deep learning capabilities could improve surgical outcomes by enhancing precision and reducing variability. This is particularly relevant in urology, where precision is critical due to the delicate nature of the procedures.
For investors, this clearance signals a potentially lucrative expansion into a high-demand specialty area. The company's ability to secure FDA approval also enhances its credibility and could lead to increased adoption of its technology in other areas. Given the annual volume of 185,000 urological procedures in the U.S., the revenue potential is substantial. However, it remains essential to monitor how well the company can penetrate this market and achieve widespread adoption among healthcare providers.
The announcement of FDA 510(k) clearance for the Senhance Surgical System in Urology is a notable development for Asensus Surgical from a financial perspective. This clearance could lead to a significant increase in U.S. sales, expanding the company's market reach. The U.S. healthcare market is substantial and the approval for both adult and pediatric urological procedures positions Asensus for potentially higher revenue streams.
The company's stock is likely to respond positively to this news as it reflects an expansion of the product's market applicability and potential revenue growth. Investors should watch for subsequent financial reports to gauge the impact on sales and profitability. An increase in sales within the U.S. market could also improve the company's overall financial health and investor confidence. Long-term, this clearance could pave the way for further product approvals and market expansions, underlining a strategic growth trajectory for the company.
The FDA clearance significantly enhances the market potential for the Senhance Surgical System in the U.S. market. The ability to perform both adult and pediatric urological procedures with this system differentiates Asensus from competitors who may not offer comparable versatility. The U.S. market for urological surgeries is substantial, with 185,000 procedures performed annually, representing a considerable revenue opportunity.
The integration of augmented intelligence and deep learning also sets the Senhance System apart, potentially leading to better outcomes and increased surgeon adoption. Market penetration will depend on effective communication of these benefits to healthcare providers and ongoing training and support. Asensus will need to focus on robust marketing strategies and partnerships with key healthcare institutions to maximize the system's uptake.
Given the favorable market conditions and the growing emphasis on advanced surgical technologies, this FDA clearance positions Asensus Surgical favorably within a competitive marketplace.
RESEARCH TRIANGLE PARK, N.C., July 23, 2024 (GLOBE NEWSWIRE) -- Asensus Surgical, Inc. (NYSE American: ASXC), a global leader of innovative digital solutions for the operating room, today announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (“FDA”) for an expanded indication to treat adult and pediatric Urology patients with the Senhance® Surgical System.
“This FDA clearance marks another milestone for Asensus Surgical and represents an additional indication expansion in the U.S. market,” said Anthony Fernando, Asensus Surgical President and CEO. “The Senhance System's precision and advanced digital capabilities make it uniquely suited for urological procedures, offering surgeons the benefit of digital tools and smaller instrumentation. We are excited to bring this technology to urologists and their patients across the United States.”
Healthcare providers perform upwards of 185,000 urological surgical procedures each year in the United States.* While the Senhance System has been successfully utilized for Urology procedures outside the U.S. for several years, this expanded indication will open the door to this important specialty to leverage the advantages of the Senhance System in the U.S. patient population.
Chester Koh MD, MBA, FACS, FAAP, a pediatric urologist and director of the Pediatric Robotic Surgery Program and Professor at Baylor College of Medicine in Houston, Texas noted that, “With a full suite of 3mm and 5mm instruments and digital integrations, the Senhance Surgical System combines the benefits of robotics and minimally invasive surgery. This is an exciting step in the evolution of Urological surgery for both adult and pediatric patients.”
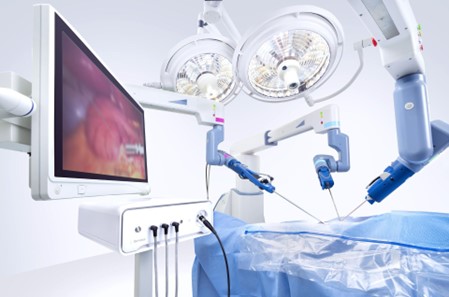
The Senhance Surgical System is designed to increase surgeon control and reduce variability through Augmented Intelligence and deep learning capabilities.
About Asensus Surgical, Inc.
Asensus Surgical is revolutionizing surgery with the first intra-operative Augmented Intelligence technology approved for use in operating rooms around the world. Recognized as an award-winning leader in digital technology, Asensus is committed to making surgery more accessible and predictable while delivering consistently superior outcomes. The Company’s novel approach to digitizing laparoscopy has led to system placements globally. Led by engineers, medical professionals, and industry luminaries, Asensus is powered by human ingenuity and driven by collaboration. To learn more about the Senhance® Surgical System and the new LUNA™ System in development, visit www.asensus.com.
Forward-Looking Statements
This press release includes statements relating to Asensus, the Senhance Surgical System and the FDA’s clearance for an expanded indication to treat adult and pediatric Urology patients with the Senhance Surgical System. These statements and other statements regarding our future plans and goals constitute "forward looking statements" within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, and are intended to qualify for the safe harbor from liability established by the Private Securities Litigation Reform Act of 1995. Such statements are subject to risks and uncertainties that are often difficult to predict, are beyond our control and which may cause results to differ materially from expectations and include whether the FDA clearance will represent another expansion in our market, and whether the Senhance Surgical System’s precision and advanced digital capabilities make it uniquely suited for urological procedures, offering surgeons the benefit of digital tools and smaller instrumentation. For a discussion of the risks and uncertainties associated with the Company’s business, please review our filings with the Securities and Exchange Commission (SEC), including our Annual Report on Form 10-K for the year ended December 31, 2023, filed with the SEC on March 21, 2024 and our other filings we make with the SEC. You are cautioned not to place undue reliance on these forward looking statements, which are based on our expectations as of the date of this press release and speak only as of the origination date of this press release. We undertake no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise.
ASENSUS SURGICAL CONTACT:
INVESTORS
Mark Klausner or Mike Vallie
ICR Westwicke
invest@asensus.com
443-213-0499
MEDIA
Dan Ventresca
Matter Communications
AsensusPR@matternow.com
617-874-5488
*Urology Market data forecasted for 2023 from DRG Clarivate Laparoscopic Market Insights - December 2021
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/de156f18-f140-4fc1-86fd-ee125390d46e








