Autonomix Medical, Inc. Reports New Positive Topline Results from First 15 Patients’ 7-Day Data Highlighting Significant Impact on Treatment of Pancreatic Cancer Pain with Maintained Pain Reduction
Autonomix Medical (NASDAQ: AMIX) reported positive results from its proof-of-concept clinical trial for pancreatic cancer pain treatment. The trial, with 60% enrollment completed (15 patients), showed a 79% responder rate among treated patients. Key findings at 7 days post-procedure include a mean 4.96 reduction in VAS pain scale scores (63% reduction), zero opioid use after 24 hours, and 66% improvement in overall health status. Femoral access patients showed significant improvement while brachial access patients showed no improvement. The trial will enroll a total of 20 patients, using the company's catheter-based microchip sensing technology for nerve detection and RF ablation.
Autonomix Medical (NASDAQ: AMIX) ha riportato risultati positivi dal suo trial clinico di proof-of-concept per il trattamento del dolore da cancro pancreatico. Il trial, con il 60% di arruolamento completato (15 pazienti), ha mostrato una percentuale di risposta del 79% tra i pazienti trattati. Le scoperte chiave a 7 giorni dopo la procedura includono una riduzione media di 4.96 nei punteggi della scala del dolore VAS (riduzione del 63%), assenza di uso di oppioidi dopo 24 ore e un miglioramento del 66% nello stato di salute generale. I pazienti con accesso femorale hanno mostrato un miglioramento significativo, mentre i pazienti con accesso brachiale non hanno mostrato miglioramenti. Il trial arruolerà un totale di 20 pazienti, utilizzando la tecnologia di sensing con microchip basata su catetere dell'azienda per la rilevazione dei nervi e l'ablazione RF.
Autonomix Medical (NASDAQ: AMIX) informó resultados positivos de su ensayo clínico de prueba de concepto para el tratamiento del dolor por cáncer de páncreas. El ensayo, con el 60% de la inscripción completada (15 pacientes), mostró una tasa de respuesta del 79% entre los pacientes tratados. Los hallazgos clave a los 7 días post-procedimiento incluyen una reducción media de 4.96 en las puntuaciones de la escala de dolor VAS (reducción del 63%), cero uso de opioides después de 24 horas y una mejora del 66% en el estado general de salud. Los pacientes con acceso femoral mostraron una mejora significativa, mientras que los pacientes con acceso braquial no mostraron mejoría. El ensayo inscribirá un total de 20 pacientes, utilizando la tecnología de detección de nervios y ablación por RF basada en catéter de la empresa.
오토노믹스 메디컬 (NASDAQ: AMIX)은 췌장암 통증 치료를 위한 개념 증명 임상 시험에서 긍정적인 결과를 보고했습니다. 이 시험은 60%의 등록이 완료된 상태로 (15명의 환자), 치료받은 환자 중 79%의 반응률을 보였습니다. 수술 후 7일의 주요 발견으로는 VAS 통증 척도에서 평균 4.96의 감소(63% 감소), 24시간 후 오피오이드 사용 제로, 그리고 전반적인 건강 상태에서 66%의 개선이 포함됩니다. 대퇴 접근 환자들은 상당한 개선을 보인 반면, 상완 접근 환자들은 개선을 보이지 않았습니다. 이번 시험은 20명의 환자를 전체적으로 모집하며, 회사의 카테터 기반 마이크로칩 감지 기술을 사용하여 신경 감지 및 RF 절제를 실시할 예정입니다.
Autonomix Medical (NASDAQ: AMIX) a rapporté des résultats positifs de son essai clinique de preuve de concept pour le traitement de la douleur liée au cancer du pancréas. L'essai, avec 60 % des inscriptions complétées (15 patients), a montré un taux de réponse de 79 % parmi les patients traités. Les principales découvertes à 7 jours après la procédure incluent une diminution moyenne de 4,96 des scores à l'échelle de douleur VAS (réduction de 63 %), aucune utilisation d'opioïdes après 24 heures et une amélioration de 66 % de l'état de santé général. Les patients ayant un accès fémoral ont montré une amélioration significative, tandis que les patients ayant un accès brachial n'ont montré aucune amélioration. L'essai recrutera un total de 20 patients, en utilisant la technologie de détection des nerfs et d'ablation RF basée sur un cathéter de l'entreprise.
Autonomix Medical (NASDAQ: AMIX) berichtete über positive Ergebnisse aus seiner Proof-of-Concept-Studie zur Behandlung von Schmerzen bei Bauchspeicheldrüsenkrebs. Die Studie, bei der 60% der Einschreibung abgeschlossen sind (15 Patienten), zeigte eine Ansprechrate von 79% unter den behandelten Patienten. Die wichtigsten Ergebnisse nach 7 Tagen nach dem Eingriff umfassen eine durchschnittliche Reduktion von 4,96 Punkten auf der VAS-Schmerzskala (63% Reduktion), keinen Opioideinsatz nach 24 Stunden und eine Verbesserung des allgemeinen Gesundheitszustands um 66%. Patienten mit femoralem Zugang zeigten signifikante Verbesserungen, während Patienten mit brachialem Zugang keine Verbesserungen zeigten. Die Studie wird insgesamt 20 Patienten einschreiben, wobei die katheterbasierte Mikrochipsensor-Technologie des Unternehmens zur Nervenerkennung und RF-Ablation verwendet wird.
- 79% responder rate (11 out of 14 treated patients)
- Significant pain reduction: 4.96 point decrease on VAS scale (63% reduction)
- Complete elimination of opioid use after 24 hours post-procedure in responding patients
- 66% improvement in overall health status for responding patients
- 60% enrollment milestone achieved with no significant adverse events
- No improvement in patients treated with brachial access
- One patient couldn't receive treatment due to severe stenosis
- Non-responders included in total treated population reduced overall efficacy to 48% pain reduction
Insights
The clinical trial results for Autonomix's nerve ablation technology show remarkable promise in treating pancreatic cancer pain. The 79% responder rate with a
The trial data reveals important technical insights: femoral access proved effective while brachial access showed no improvement, providing important guidance for future procedure protocols. The
The amended protocol's focus on tumor encroachment and bio-measurements will provide valuable data for optimizing patient selection and treatment effectiveness. With
This data represents a significant market opportunity in the $4.3 billion global pancreatic cancer treatment market. With over
The technology's superior sensitivity (3,000 times greater than current options) and precision-guided approach create substantial barriers to entry. The rapid enrollment pace suggests strong physician interest and market demand. For a company with a market cap of
Company reaches
Clinically meaningful mean of 4.96 or
THE WOODLANDS, TX, Oct. 31, 2024 (GLOBE NEWSWIRE) -- Autonomix Medical, Inc. (NASDAQ: AMIX) (“Autonomix” or the “Company”), a medical device company focused on advancing innovative technologies to revolutionize how diseases involving the nervous system are diagnosed and treated, today announced preliminary positive results from the first 15 patients in the Company’s ongoing proof-of-concept human clinical trial (the “Trial”) evaluating the safety and effectiveness of delivering transvascular energy to ablate relevant problematic nerves and mitigate pain in patients with pancreatic cancer pain.
“The significant reduction in pain and improvement in quality of life demonstrated in the study to date are incredibly encouraging. These data provide a strong indication that our technology has the potential to revolutionize the treatment paradigm for pancreatic cancer pain,” Brad Hauser, CEO of Autonomix commented. “Additionally, achieving
Chief Medical Officer of Autonomix, Dr. Robert Schwartz added, “Pain has a significant impact on the lives of patients with pancreatic cancer. Available data suggests that over
Summary of First 15 Patients’ Topline Results 7-Days Post-Procedure
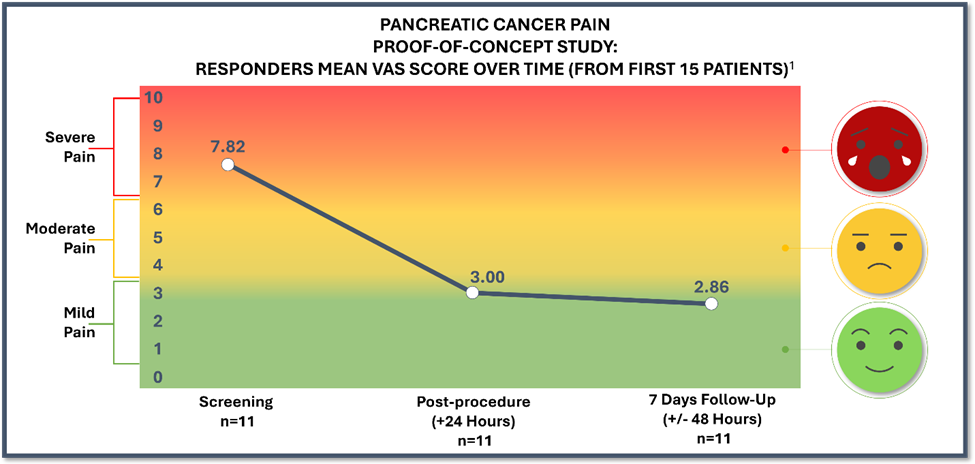
- 11 patients were treated with femoral access and three (3) were treated with brachial access. Patients treated with brachial access showed no improvement in their pain scores while patients treated with femoral access did respond to treatment. One (1) patient could not receive treatment due to a more severe stenosis than what appeared on pre-screening scans and is not included in the modified intent to treat population. The results presented in the chart above are for the 11 patients in the responder group.
79% of patients responded (11 out of the 14 treated patients) with a mean 4.96 reduction of pain on the VAS pain scale (from baseline of 7.82 to 2.86) at 7 days post-procedure.- Study through 7 days post-procedure showed a decrease in opioid demand and no responding patient needed dose increase; No responding patient needed opioids after their 24-hour post-procedure follow-up visit.
- Responding patients reported a mean
66% improvement in overall health status at 7 days post-procedure.
When evaluating the total treated population, including non-responders, the mean reduction in the VAS pain score was a 3.64 point reduction, or
As previously announced, Autonomix amended the Trial protocol to include the gathering of additional information on tumor encroachment on the vessels, as well as other key bio-measurements that may correlate with effective nerve ablation. Additionally, the Company has further defined severe pain for inclusion criteria as a 7 or above on the VAS scale as indicated by the patient rather than physician determination. A total of twenty (20) patients will be enrolled in the Trial that will be formally included in the Trial data results and analysis of Trial objectives. Suitability is determined by the primary oncologist caring for the patients with the treating Principal Investigator (“PI”) confirming eligibility for the Trial.
The Company’s first-in-class technology platform utilizes a catheter-based microchip sensing array antenna that has the ability to detect and differentiate neural signals with up to 3,000 times greater sensitivity than currently available technologies. Once target nerves are identified, Autonomix uses its proprietary radio frequency (RF) ablation technology to kill targeted nerves, enabling a precision guided sense, treat and verify approach to addressing a number of disease categories from chronic pain management to hypertension and cardiology. Current approaches, primarily relying on opioids or invasive ethanol injections, can provide only limited relief and may lead to risky side effects. For more information about the Company’s technology, please visit autonomix.com.
About the Trial
The goal of the Trial is to assess pain reduction via radiofrequency (RF) ablation. The Company’s catheter-based microchip sensing array used to detect and differentiate neural signaling was not used in this trial and will be evaluated in future studies.
The first five patients were enrolled and treated according to protocol in the beginning of the Trial to familiarize the PI with the procedure and will not be included in the analysis of the Trial objectives. These first five “lead-in” patients successfully completed the procedure per protocol with no immediate procedural-related complications or significant adverse events.
The primary objective of the Trial is to assess the success rate of ablating relevant nerves to mitigate pain in patients with pancreatic cancer pain utilizing RF ablation in a transvascular approach to the nerves in the region. Secondary objectives include assessing the incidence of device- and procedure-related adverse events up to 4-6 weeks post-procedure; estimating the change in pain levels from pre- to post-procedure; and estimating the change in quality of life from pre- to post-procedure. All patients who have had a successful procedure will be evaluated at 7 days, 4-6 weeks, and at 3 months post-procedure. All patients entered the study with severe abdominal pain from unresectable pancreatic cancer and a life expectancy of 3 months or less. Following the successful completion of the procedure, five patients have since succumbed to their disease. Both events were expected outcomes and not related to the Trial procedure.
About Autonomix Medical, Inc.
Autonomix is a medical device company focused on advancing innovative technologies to revolutionize how diseases involving the nervous system are diagnosed and treated. The Company’s first-in-class platform system technology includes a catheter-based microchip sensing array that may have the ability to detect and differentiate neural signals with approximately 3,000 times greater sensitivity than currently available technologies. We believe this will enable, for the first time ever, transvascular diagnosis and treatment of diseases involving the peripheral nervous system virtually anywhere in the body.
We are initially developing this technology for the treatment of pain, with initial trials focused on pancreatic cancer, a condition that causes debilitating pain and is without a reliable solution. Our technology constitutes a platform to address dozens of indications, including cardiology, hypertension and chronic pain management, across a wide disease spectrum. Our technology is investigational and has not yet been cleared for marketing in the United States.
For more information, visit autonomix.com and connect with the Company on X, LinkedIn, Instagram and Facebook.
Forward Looking Statements
Some of the statements in this release are “forward-looking statements,” which involve risks and uncertainties. Forward-looking statements in this press release include, without limitation, the potential of the technology to treat pain associated with pancreatic cancer, to successfully enroll patients within the specific timeframe, and to complete its clinical study in pancreatic cancer pain. Such forward-looking statements can be identified by the use of words such as “should,” “might,” “may,” “intends,” “anticipates,” “believes,” “estimates,” “projects,” “forecasts,” “expects,” “plans,” and “proposes.”
Although Autonomix believes that the expectations reflected in these forward-looking statements are based on reasonable assumptions, there are a number of risks and uncertainties that could cause actual results to differ materially from such forward-looking statements. You are urged to carefully review and consider any cautionary statements and other disclosures, including the statements made under the heading “Risk Factors” and elsewhere in the Annual Report on Form 10-K filed with the U.S. Securities and Exchange Commission (SEC) on May 31, 2024. Forward-looking statements speak only as of the date of the document in which they are contained and Autonomix does not undertake any duty to update any forward-looking statements except as may be required by law.
Investor and Media Contact
JTC Team, LLC
Jenene Thomas
908.824.0775
autonomix@jtcir.com
1 First 15 subjects includes the five lead-in subjects, which will not be included in the final data release
2 Westermann A, Matrisian LM, Rahib L. The need for improvement in the management of fatigue, depression and pain in pancreatic cancer. J Clin Oncol 2019;37(suppl 4):429a.
Attachments

FAQ
What were the pain reduction results in Autonomix Medical's (AMIX) pancreatic cancer trial?
How many patients have been enrolled in Autonomix Medical's (AMIX) clinical trial?
Did Autonomix Medical's (AMIX) treatment eliminate opioid use in pancreatic cancer patients?







