IZERVAY™ (avacincaptad pegol intravitreal solution) Monthly or Every Other Month Reduced Geographic Atrophy Lesion Growth Through 2 Years
- None.
- None.
Phase 3 results presented at the AAO Annual Meeting demonstrated the IZERVAY treatment effect more than doubled over 2 years compared to 1 year
IZERVAY safety profile was consistent with year 1, with no cases of ischemic optic neuropathy or retinal vasculitis
Over 2 years, only a slight increased incidence of choroidal neovascularization was observed for IZERVAY pooled vs. sham; in year 2, incidence of CNV was similar for IZERVAY EOM vs. sham
Key study results include:
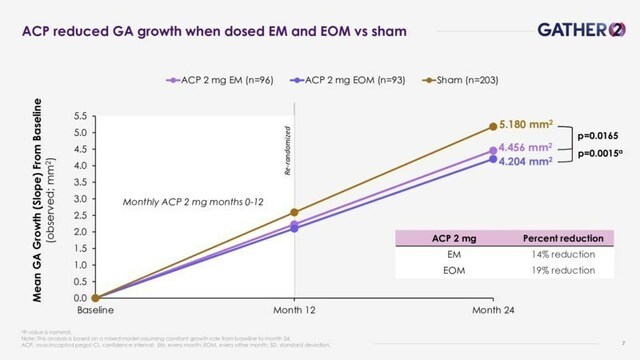
- IZERVAY dosed EM through 2 years demonstrated a statistically significant year-over-year reduction of
14% in the mean rate of GA growth at 2 years from baseline vs. sham (p=0.0165), the primary objective of the year 2 analyses from GATHER2. - EOM dosing of IZERVAY, after a year of monthly dosing, resulted in a reduction of
19% in the mean GA growth rate at 2 years vs. sham (nominal p-value=0.0015). - IZERVAY treatment effect more than doubled over 2 years compared to 1 year.
- The prespecified objective of demonstrating that IZERVAY reduced the rate of ≥15-letter persistent vision loss compared to sham over 2 years was not statistically significant. Persistent vision loss will be further explored across several sensitivity analyses.
- IZERVAY was well tolerated over 2 years, with one case each of non-serious intraocular inflammation (IOI) and culture-positive endophthalmitis. There were no cases of ischemic neuropathy or retinal vasculitis.
- Over 2 years, only a slight increased incidence of choroidal neovascularization (CNV) was observed for IZERVAY pooled vs. sham. In year 2, the incidence of CNV was similar for IZERVAY EOM vs. sham.
Arshad M. Khanani, MD, MA, FASRS, Director of Clinical Research at Sierra Eye Associates,
"GA is a debilitating, progressive disease that can impact patients' independence. These exciting results demonstrate year-over-year reductions in the rate of GA lesion growth in patients treated with monthly and every-other-month dosing compared to sham. The GA treatment benefit was observed as early as six months and continued to increase over time. The safety profile over 2 years was consistent with year 1, with no new safety signals identified. These results further validate that IZERVAY is an effective and safe treatment option for patients with GA."
Dhaval Desai, Senior Vice President and Chief Development Officer, Iveric Bio, An Astellas Company
"We are pleased with these new results and look forward to sharing the findings with regulatory health authorities. Thank you to all the patients and their families, as well as the eye care professionals who participated in the GATHER2 study. Patients are at the center of all we do, and Astellas is committed to advancing research and developing transformational therapies for people living with retinal diseases."
Treatment-emergent adverse events (TEAEs) over 2 years were similar and consistent with year 1. Ocular TEAEs were observed in
- The three most common ocular TEAEs in patients treated with IZERVAY vs. sham over 2 years were conjunctival hemorrhage (
16.9% vs.9.0% ), intraocular pressure increased (13.3% vs.0.9% ) and CNV (11.6% vs.9.0% ). - Serious ocular TEAEs over 2 years were equivalent between the IZERVAY and sham study groups (
1.8% vs.0.9% ). In year 2, there was one case of culture-positive endophthalmitis and one case of subluxated intraocular lens for IZERVAY compared to no such cases in the sham group.
IZERVAY was approved by the
Astellas has already reflected the impact from this result in its financial forecast of the current fiscal year ending March 31, 2024.
About the GATHER2 Clinical Trial
GATHER2 (NCT04435366) was a randomized, double-masked, sham-controlled, multicenter Phase 3 clinical trial to evaluate the safety and efficacy of intravitreal administration of avacincaptad pegol (ACP) in 448 enrolled patients with GA secondary to AMD. ACP met its primary objective at 12 months, for which patients were randomized to receive either ACP or sham procedure monthly. In year 2 of the study, patients treated with ACP in year 1 were re-randomized to receive either ACP dosed monthly (EM, n=96) or every other month (EOM, n=93); patients who received sham in year 1 continued to receive sham in year 2 (n=203). IZERVAY is continuing to be evaluated in an open-label extension study.
About IZERVAY™ (avacincaptad pegol intravitreal solution)
IZERVAY (avacincaptad pegol intravitreal solution) is indicated for the treatment of geographic atrophy (GA) secondary to age-related macular degeneration (AMD).
IMPORTANT
CONTRAINDICATIONS
- IZERVAY is contraindicated in patients with ocular or periocular infections and in patients with active intraocular inflammation.
WARNINGS AND PRECAUTIONS
- Endophthalmitis and Retinal Detachments
- Intravitreal injections, including those with IZERVAY, may be associated with endophthalmitis and retinal detachments. Proper aseptic injection technique must always be used when administering IZERVAY in order to minimize the risk of endophthalmitis. Patients should be instructed to report any symptoms suggestive of endophthalmitis or retinal detachment without delay and should be managed appropriately.
- Neovascular AMD
- In clinical trials, use of IZERVAY was associated with increased rates of neovascular (wet) AMD or choroidal neovascularization (
7% when administered monthly and4% in the sham group) by Month 12. Patients receiving IZERVAY should be monitored for signs of neovascular AMD.
- In clinical trials, use of IZERVAY was associated with increased rates of neovascular (wet) AMD or choroidal neovascularization (
- Increase in Intraocular PressureTransient increases in intraocular pressure (IOP) may occur after any intravitreal injection, including with IZERVAY. Perfusion of the optic nerve head should be monitored following the injection and managed appropriately.
ADVERSE REACTIONS
- Most common adverse reactions (incidence ≥
5% ) reported in patients receiving IZERVAY were conjunctival hemorrhage, increased IOP, blurred vision, and neovascular age-related macular degeneration.
- Most common adverse reactions (incidence ≥
Please see full Prescribing Information for more information.
About Geographic Atrophy
Age-related macular degeneration (AMD) is the major cause of moderate and severe loss of central vision in aging adults, affecting both eyes in the majority of patients. The macula is a small area in the central portion of the retina responsible for central vision. As AMD progresses, the loss of retinal cells and the underlying blood vessels in the macula results in marked thinning and/or atrophy of retinal tissue. Geographic atrophy, associated with AMD, leads to further irreversible loss of vision in these patients.
About Astellas
Astellas Pharma Inc. is a pharmaceutical company conducting business in more than 70 countries around the world. We are promoting the Focus Area Approach that is designed to identify opportunities for the continuous creation of new drugs to address diseases with high unmet medical needs by focusing on Biology and Modality. Furthermore, we are also looking beyond our foundational Rx focus to create Rx+® healthcare solutions that combine our expertise and knowledge with cutting-edge technology in different fields of external partners. Through these efforts, Astellas stands on the forefront of healthcare change to turn innovative science into VALUE for patients. For more information, please visit our website at https://www.astellas.com/en.
About Iveric Bio
Iveric Bio, An Astellas Company, is a science-driven biopharmaceutical company focused on the discovery and development of novel treatments for retinal diseases with significant unmet medical needs. The Company is committed to having a positive impact on patients' lives by delivering high-quality, safe, and effective treatments designed to address debilitating retinal diseases including earlier stages of age-related macular degeneration. For more information on the Company, please visit www.ivericbio.com.
Cautionary Notes
In this press release, statements made with respect to current plans, estimates, strategies and beliefs and other statements that are not historical facts are forward-looking statements about the future performance of Astellas. These statements are based on management's current assumptions and beliefs in light of the information currently available to it and involve known and unknown risks and uncertainties. A number of factors could cause actual results to differ materially from those discussed in the forward-looking statements. Such factors include, but are not limited to: (i) changes in general economic conditions and in laws and regulations, relating to pharmaceutical markets, (ii) currency exchange rate fluctuations, (iii) delays in new product launches, (iv) the inability of Astellas to market existing and new products effectively, (v) the inability of Astellas to continue to effectively research and develop products accepted by customers in highly competitive markets, and (vi) infringements of Astellas' intellectual property rights by third parties.
Information about pharmaceutical products (including products currently in development) which is included in this press release is not intended to constitute an advertisement or medical advice.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/izervay-avacincaptad-pegol-intravitreal-solution-monthly-or-every-other-month-reduced-geographic-atrophy-lesion-growth-through-2-years-301977771.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/izervay-avacincaptad-pegol-intravitreal-solution-monthly-or-every-other-month-reduced-geographic-atrophy-lesion-growth-through-2-years-301977771.html
SOURCE ASTELLAS PHARMA INC.
FAQ
What are the key results from the GATHER2 trial for IZERVAY?
What are the common ocular TEAEs associated with IZERVAY?