EMA Recommends Arpraziquantel for Treatment of Schistosomiasis in Preschool-Aged Children
- Astellas contributed to the development of a pediatric formulation to treat schistosomiasis
- The new pediatric formulation of arpraziquantel is palatable for very young children and is administered via a 150 mg dispersible tablet
- The positive CHMP scientific opinion by EMA of arpraziquantel represents a significant milestone in alignment with Astellas' Access to Health focus
- None.
- Astellas contributed to the development of a pediatric formulation to treat schistosomiasis as a member of the Pediatric Praziquantel Consortium -
Astellas provided its innovative formulation technology to co-develop arpraziquantel as a member of the Consortium. The new pediatric formulation of arpraziquantel is palatable for very young children by reducing the bitter taste associated with the medication and administered via a 150 mg dispersible tablet that is dissolved in water. The prototype of the pediatric formulation has been developed by Astellas and further optimized by Merck (
In parallel with this regulatory work, the Consortium's implementation research program (ADOPT)*2 is ongoing and preparing for the introduction of arpraziquantel in the first endemic countries in
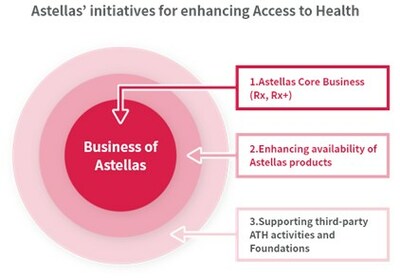
Astellas has set a Strategic Goal to "deepen our engagement in sustainability" in the Astellas Corporate Strategic Plan 2021. To address the key sustainability issue of Access to Health (ATH), Astellas uses a comprehensive approach including, (1) Astellas' core business (Rx, Rx+), (2) Enhancing availability of Astellas products (Access to Medicines), and (3) Collaboration and support for the activities implemented by external partners. Initiatives in the Consortium apply to (3), and the positive CHMP scientific opinion by EMA of arpraziquantel represents a significant milestone in alignment with Astellas' Access to Health focus.
Naoki Okamura, President and CEO, Astellas
"We are so pleased that arpraziquantel received a positive scientific opinion by EMA, and also proud that Astellas could contribute to its development by providing our innovative formulation technologies. Access to Health is essential to the health of people around the world, and we recognize it as a material issue for Astellas. Through our VISION to be "On the forefront of healthcare change to turn innovative science into VALUE for patients", we are proactively taking a comprehensive approach to solving this issue."
For more information on Astellas' contribution to the development of arpraziquantel, please see (https://www.astellas.com/jp/en/sustainability/development-of-pediatric-formulation-for-schistosomiasis).
*1 EU-M4all: EU-M4all is the procedure which aims to facilitate patient access to high-priority medicines for human use intended for countries outside EU. Through the EU-M4all procedure, EMA's CHMP assess and can provide scientific opinions on high-priority human medicines, including vaccines. Together with the positive scientific opinion, the prequalification by World Health Organization (WHO) will support the regulatory pathway in the target countries. For more detail, please refer to EMA's website. |
*2 ADOPT: Adoption of Levo-Praziquantel 150 mg for schistosomiasis by endemic countries |
About schistosomiasis
Schistosomiasis (also known as bilharzia) is one of the most prevalent parasitic diseases worldwide and a very important one in terms of public health burden and economic impact. It is a poverty-related disease that is widespread in tropical and subtropical regions where large sections of the population have no access to clean water. Flatworms transmit the disease and people become infected with the parasite through contact with freshwater, for example, while working, swimming, fishing, or washing their clothes. The minuscule larvae penetrate human skin, enter the blood vessels, and attack internal organs. The infection rate is particularly high among children. Schistosomiasis is a chronic condition and is classified by the World Health Organization (WHO) as one of 20 neglected tropical diseases (NTDs).
About Arpraziquantel
The current standard of care treatment for schistosomiasis is praziquantel. Praziquantel is safe, effective, and suitable for school-aged children and adults. Extending the range of options for the treatment of schistosomiasis, arpraziquantel is tailored for preschool-aged children against Schistosoma mansoni and Schistosoma haematobium. Tested in clinical development, under the responsibility of Merck, arpraziquantel contains the pharmacologically active enantiomer of praziquantel. In developing arpraziquantel, the Pediatric Praziquantel Consortium established a pediatric drug development program, divided into four major steps: preclinical development, clinical development, registration, and access. All details can be found on the Consortium website.
About the Pediatric Praziquantel Consortium
The Pediatric Praziquantel Consortium is an international public-private partnership that aims to reduce the global disease burden of schistosomiasis and improve child health by addressing the medical needs of infected preschool-aged children. Its mission is to develop, register, and provide access to a suitable pediatric drug for treating schistosomiasis in children 3 months to 6 years of age. For more information, and to see an overview of all Consortium partners, visit the Consortium website.
Consortium Partners
- Merck (
Germany ) - Astellas Pharma Inc. (
Japan ) - The Swiss Tropical and Public Health Institute (
Switzerland ) - Lygature (
The Netherlands ) - Farmanguinhos (
Brazil ) - Unlimit Health (
United Kingdom ) - Kenya Medical Research Institute (
Kenya ) - Université Félix Houphouët-Boigny (Côte d'Ivoire)
- Klinikum rechts der Isar der Technischen Universität München (
Germany ) - Ministry of Health Côte d'Ivoire (Côte d'Ivoire)
- African Institute for Health and Development (
Kenya )
Other collaborators that contribute to the mission of the Pediatric Praziquantel Consortium:
- Makerere University School of Public Health (
Uganda ) - Ministry of Health Kenya, Division of Vector Borne and NTDs (
Kenya ) - Ministry of Health Uganda, Vector Borne and NTDs Control Division (
Uganda )
About Astellas
Astellas Pharma Inc. is a pharmaceutical company conducting business in more than 70 countries around the world. We are promoting the Focus Area Approach that is designed to identify opportunities for the continuous creation of new drugs to address diseases with high unmet medical needs by focusing on Biology and Modality. Furthermore, we are also looking beyond our foundational Rx focus to create Rx+® healthcare solutions that combine our expertise and knowledge with cutting-edge technology in different fields of external partners. Through these efforts, Astellas stands on the forefront of healthcare change to turn innovative science into VALUE for patients. For more information, please visit our website at https://www.astellas.com/en.
Cautionary Notes
In this press release, statements made with respect to current plans, estimates, strategies and beliefs and other statements that are not historical facts are forward-looking statements about the future performance of Astellas. These statements are based on management's current assumptions and beliefs in light of the information currently available to it and involve known and unknown risks and uncertainties. A number of factors could cause actual results to differ materially from those discussed in the forward-looking statements. Such factors include, but are not limited to: (i) changes in general economic conditions and in laws and regulations, relating to pharmaceutical markets, (ii) currency exchange rate fluctuations, (iii) delays in new product launches, (iv) the inability of Astellas to market existing and new products effectively, (v) the inability of Astellas to continue to effectively research and develop products accepted by customers in highly competitive markets, and (vi) infringements of Astellas' intellectual property rights by third parties.
Information about pharmaceutical products (including products currently in development) which is included in this press release is not intended to constitute an advertisement or medical advice.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/ema-recommends-arpraziquantel-for-treatment-of-schistosomiasis-in-preschool-aged-children-302017407.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/ema-recommends-arpraziquantel-for-treatment-of-schistosomiasis-in-preschool-aged-children-302017407.html
SOURCE Astellas Pharma Inc.
FAQ
What did Astellas contribute to the development of a pediatric formulation for schistosomiasis?
What is the significance of the positive CHMP scientific opinion for arpraziquantel?