ADOCIA Announces Exceptional Weight Loss for Obese People with Type 1 Diabetes using M1Pram in a post-hoc analysis
Adocia has announced positive results from its Phase 2 study of M1Pram for obese patients with type 1 diabetes. Patients experienced an average weight loss of 5.5 kg at 16 weeks, significantly more than those on Humalog®. M1Pram also provided comparable glycemic control, maintaining HbA1c levels and Time-In-Range. The study indicated better appetite control in 82.4% of M1Pram users. Notably, M1Pram could reduce daily prandial insulin doses by 21%, marking it as a potential treatment option for obesity in type 1 diabetes.
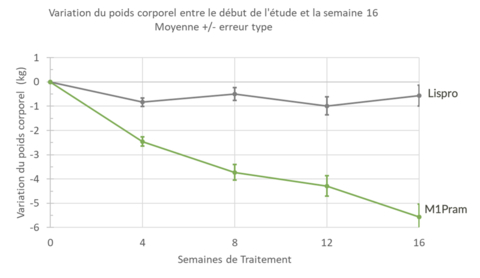
- Weight loss of 5.5 kg in 16 weeks for obese type 1 diabetes patients using M1Pram, compared to 0.57 kg with Humalog.
- M1Pram demonstrated equivalent glycemic control to Humalog, maintaining HbA1c levels.
- Better appetite control reported by 82.4% of M1Pram patients versus 43.2% for Humalog.
- Reduction of daily prandial insulin dose by 21% in the general study population.
- M1Pram has patent protection until 2038, enhancing market exclusivity opportunities.
- Higher total adverse events reported for M1Pram (76) compared to Humalog (38), primarily due to gastrointestinal side effects.
- A weight loss of 5.5kg measured at 16 weeks of treatment in people with type 1 diabetes and a body mass index greater than 30kg/m2
- Glycemic control efficacy comparable to that obtained with the commercial reference insulin Humalog® (Eli Lilly)
-
These results were presented at the 58th
European Association for the Study of Diabetes (EASD) Annual Meeting held inStockholm fromSeptember 19 to 23, 2022
(Graphic: Business Wire)
M1PRAM, ADDRESSING THE UNMET MEDICAL NEED OF OBESITY IN PEOPLE WITH TYPE 1 DIABETES
Post-hoc analyses revealed the greater efficacy of M1Pram in a subpopulation of obese patients with a Body Mass Index (BMI) greater than 30kg/m2. Weight loss in the M1Pram arm was -5.56kg versus -0.57 kg (p=0.03) in the Humalog arm at week 16, and weight loss had not plateaued by the end of the study.
The satisfaction questionnaire clearly demonstrated better appetite control with M1Pram for
"Given that more and more people with type 1 diabetes are overweight or obese, the availability of M1Pram allows these patients to maintain glycemic control while losing weight, an important outcome”, declared
As a reminder, the CT041 Phase 2 clinical trial was comparing M1Pram to insulin lispro (Humalog®, Eli Lilly). The positive results on the total population were communicated on
WHILE REDUCING WEIGHT, M1PRAM OFFERS GLYCEMIC CONTROL AS GOOD AS GOLD STANDARD MEALTIME INSULIN
M1Pram demonstrated to be equivalent to Humalog in controlling blood glucose, as safe in terms of risk of hypoglycemia and as convenient in terms of use.
-
Both treatments maintain HbA1c levels and Time-In-Range in patients with a mean HbA1c level of
7.4% at baseline - The number and severity of hypoglycemic events are similar in both treatment arms
- This coformulation is injected at mealtime by single injection
In addition, M1Pram reduced the daily dose of prandial insulin by
M1Pram had an overall good safety profile. The difference in total adverse events of M1Pram versus Humalog (76 vs. 38) was mainly due to gastrointestinal side effects as documented in the pramlintide literature.
OBESITY, A
Nine million people in the world currently suffer from type 1 diabetes and this figure will double in the coming years2.
Moreover, emerging evidence suggests that obesity contributes to insulin resistance, dyslipidemia, and cardiometabolic complications in type 1 diabetes5.
To date, pramlintide is the only product reducing weight that is approved by the FDA as an adjunct to insulin for people with type 1 diabetes.
M1PRAM TO REPLACE MEALTIME INSULINS FOR OBESE PEOPLE WITH DIABETES
M1Pram combines M1 insulin and pramlintide in a regular insulin pen. M1Pram coformulation is patented by
Pramlintide is an amylin analog that is FDA approved as an adjunct to insulin in type 1 and type 2 diabetes. Pramlintide has demonstrated having significant effects in improving glycemic control, weight loss in overweight patients and well-being. Despite its clinical benefits, pramlintide has never been largely used by patients because it requires 3 additional injections on top of insulin daily injections, these two hormones normally being incompatible in one formulation. Based on 15-years of experience in protein formulation and diabetes,
“At the EASD Annual Meeting, the uniqueness of M1Pram, mealtime insulin allowing significant weight loss, attracted the interest of several companies involved in the treatment of diabetes. Our goal now is to establish a strategic partnership to continue the development of M1Pram in obese people with type 1 Diabetes and extend it to obese people with type 2”, declared
About
1) The BioChaperone® technology for the development of new generation insulins and products combining insulins with other classes of hormones; 2) AdOral®, an oral peptide delivery technology; 3) AdoShell®, an immunoprotective biomaterial for cell transplantation with a first application in pancreatic cells transplantation for patients with "brittle" diabetes.
Based in
Disclaimer
This press release contains certain forward-looking statements concerning
The forward-looking statements contained in this press release are also subject to risks not yet known to
1https://www.adocia.com/wp-content/uploads/2022/06/PR-Adocia-M1Pram-Topline-Results-ENG.pdf
2 Type 1 Diabetes Index https://www.t1dindex.org/
3 Conway B, Miller RG, Costacou T, Fried L, Kelsey S, Evans RW, Orchard TJ. Temporal patterns in overweight and obesity in Type 1 diabetes. Diabet Med. 2010 Apr;27(4):398-404. doi: 10.1111/j.1464-5491.2010.02956.x. PMID: 20536510; PMCID: PMC3129711.
4 Amelia S Wallace, Alex R Chang,
5Corbin KD, Driscoll KA, Pratley RE, Smith SR, Maahs DM, Mayer-Davis EJ; Advancing Care for Type 1 Diabetes and Obesity Network (ACT1ON). Obesity in Type 1 Diabetes: Pathophysiology, Clinical Impact, and Mechanisms. Endocr Rev. 2018
View source version on businesswire.com: https://www.businesswire.com/news/home/20221005005658/en/
CEO
contactinvestisseurs@adocia.com
Tel : +33 (0)4 72 610 610
www.adocia.com
Ulysse Communication
Margaux Puech Pays d’Alissac
adocia@ulysse-communication.com
+ 33 (0)6 64 79 97 51
Source:
FAQ
What were the results of the Adocia Phase 2 study for M1Pram?
How does M1Pram compare to Humalog in terms of glycemic control?
What percentage of patients reported better appetite control with M1Pram?
Is there any patent protection for M1Pram?







