Xtant Medical Expands Product Portfolio with Launch of its own Viable Bone Matrix, OsteoVive(R) Plus
Xtant Medical Holdings, Inc. (NYSE American: XTNT) has launched OsteoVive Plus, a new moldable, viable bone matrix for various grafting procedures. This product expands Xtant's portfolio and is now available to their nationwide distribution network. OsteoVive Plus features Advanced Purloc™ Fiber Technology, offering a high concentration of elongated cortical fibers for optimal surgical placement.
The launch aligns with Xtant's strategy to drive margin improvement and generate positive operating cash flow. Manufactured at their Belgrade facility, OsteoVive Plus reinforces Xtant's position as an innovative medical technology company. The product will be showcased at the North American Spine Society (NASS) 2024 annual meeting from September 25-28 in Chicago.
Xtant Medical Holdings, Inc. (NYSE American: XTNT) ha lanciato OsteoVive Plus, una nuova matrice ossea moldabile e vitale per vari interventi di innesto. Questo prodotto arricchisce il portafoglio di Xtant ed è ora disponibile nella loro rete di distribuzione nazionale. OsteoVive Plus presenta la tecnologia Advanced Purloc™ Fiber, che offre un'alta concentrazione di fibre corticali allungate per un posizionamento chirurgico ottimale.
Il lancio è in linea con la strategia di Xtant di migliorare i margini e generare un flusso di cassa operativo positivo. Prodotto presso il loro stabilimento di Belgrado, OsteoVive Plus rafforza la posizione di Xtant come azienda innovativa nel settore della tecnologia medica. Il prodotto sarà presentato al congresso annuale della North American Spine Society (NASS) 2024 che si terrà dal 25 al 28 settembre a Chicago.
Xtant Medical Holdings, Inc. (NYSE American: XTNT) ha lanzado OsteoVive Plus, una nueva matriz ósea moldeable y viable para diversos procedimientos de injerto. Este producto amplía el portafolio de Xtant y ya está disponible en su red de distribución nacional. OsteoVive Plus cuenta con la Tecnología de Fibra Advanced Purloc™, que ofrece una alta concentración de fibras corticales alargadas para una colocación quirúrgica óptima.
El lanzamiento está alineado con la estrategia de Xtant de mejorar los márgenes y generar un flujo de caja operativo positivo. Fabricado en su instalación de Belgrado, OsteoVive Plus refuerza la posición de Xtant como una empresa innovadora en tecnología médica. El producto se presentará en la reunión anual de la North American Spine Society (NASS) 2024, que se llevará a cabo del 25 al 28 de septiembre en Chicago.
Xtant Medical Holdings, Inc. (NYSE American: XTNT)가 다양한 이식 절차를 위한 새로운 형태의 생체 골 매트릭스인 OsteoVive Plus를 출시했습니다. 이 제품은 Xtant의 포트폴리오를 확장하며, 이제 전국 배급 네트워크를 통해 제공됩니다. OsteoVive Plus는 최적의 수술적 배치를 위한 고농도의 길쭉한 피질 섬유를 제공하는 Advanced Purloc™ Fiber Technology를 특징으로 하고 있습니다.
이번 출시를 통해 Xtant는 마진 개선과 긍정적인 운영 현금 흐름을 창출하겠다는 전략을 강화합니다. 벨그라드 시설에서 제조된 OsteoVive Plus는 혁신적인 의료 기술 회사로서의 Xtant의 입지를 강화합니다. 이 제품은 2024년 9월 25일부터 28일까지 시카고에서 열리는 북미 척추학회(NASS) 연례 회의에서 소개될 예정입니다.
Xtant Medical Holdings, Inc. (NYSE American: XTNT) a lancé OsteoVive Plus, une nouvelle matrice osseuse moldable et viable pour divers procédés de greffe. Ce produit élargit le portefeuille d'Xtant et est désormais disponible dans leur réseau de distribution national. OsteoVive Plus utilise la technologie Advanced Purloc™ Fiber, offrant une forte concentration de fibres corticales allongées pour un placement chirurgical optimal.
Le lancement s'aligne avec la stratégie d'Xtant pour améliorer les marges et générer un flux de trésorerie opérationnel positif. Fabriqué dans leur installation de Belgrade, OsteoVive Plus renforce la position d'Xtant en tant qu'entreprise innovante dans le domaine de la technologie médicale. Le produit sera présenté lors de la réunion annuelle de la North American Spine Society (NASS) 2024, qui se tiendra du 25 au 28 septembre à Chicago.
Xtant Medical Holdings, Inc. (NYSE American: XTNT) hat OsteoVive Plus eingeführt, eine neue formbare, vitale Knochenmatrix für verschiedene Transplantationsverfahren. Dieses Produkt erweitert das Portfolio von Xtant und ist jetzt über ihr nationales Vertriebsnetz erhältlich. OsteoVive Plus verfügt über die Advanced Purloc™ Fiber Technology, die eine hohe Konzentration an verlängerten kortikalen Fasern für eine optimale chirurgische Platzierung bietet.
Die Einführung steht im Einklang mit der Strategie von Xtant, die Margen zu verbessern und einen positiven operativen Cashflow zu generieren. Hergestellt in ihrer Einrichtung in Belgrad, stärkt OsteoVive Plus die Position von Xtant als innovatives Medizintechnologie-Unternehmen. Das Produkt wird auf dem jährlichen Treffen der North American Spine Society (NASS) 2024 vom 25. bis 28. September in Chicago präsentiert.
- Launch of new product OsteoVive Plus, expanding market opportunities
- Product manufactured in-house at Belgrade facility, potentially improving margins
- Aligns with strategy to drive margin improvement and generate positive operating cash flow
- Reinforces company's position as an innovative medical technology company
- Product available through nationwide distribution network and OEM relationships
- None.
Insights
Xtant Medical's launch of OsteoVive Plus, a new viable bone matrix, represents a significant advancement in cellular bone technology. The product's Advanced Purloc™ Fiber Technology offers a high concentration of elongated cortical fibers, potentially improving surgical outcomes in grafting procedures. This innovation could give Xtant a competitive edge in the
The launch of OsteoVive Plus aligns with Xtant Medical's strategy to drive margin improvement and generate positive operating cash flow. In-house manufacturing at their Belgrade facility could potentially lead to better cost control and higher profit margins. However, the financial impact remains uncertain without specific revenue projections or pricing information. The expanded product portfolio may open new market opportunities, potentially increasing the company's market share in the spinal disorder treatment sector. Investors should monitor upcoming quarterly reports for early indications of OsteoVive Plus's contribution to revenue growth and margin expansion. The product's success could be a catalyst for Xtant's stock performance, but it's important to see concrete financial results before drawing definitive conclusions.
The launch of OsteoVive Plus could strengthen Xtant Medical's position in the competitive bone graft substitutes market. The product's moldable and cohesive properties address surgeons' needs for ease of use and precise placement. Distribution through a nationwide network of independent agents and OEM relationships may facilitate rapid market penetration. However, success will depend on factors such as pricing strategy, reimbursement policies and the product's performance compared to existing alternatives. The upcoming NASS annual meeting provides a important platform for showcasing OsteoVive Plus to key stakeholders. Market reception at this event could be an early indicator of the product's potential. While the launch is promising, it's essential to monitor adoption rates and gather feedback from early users to gauge long-term market impact.
New Cellular Bone Matrix Designed with Cohesive and Moldable Handling Characteristics
BELGRADE, MT / ACCESSWIRE / September 18, 2024 / Xtant Medical Holdings, Inc. (NYSE American:XTNT), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, today announced it has launched a new addition to its robust product portfolio, OsteoVive Plus, a moldable, viable bone matrix that can be used in a variety of grafting procedures. The graft is now available, to the company's nationwide distribution network of independent agents as well as original equipment manufacturer relationships.
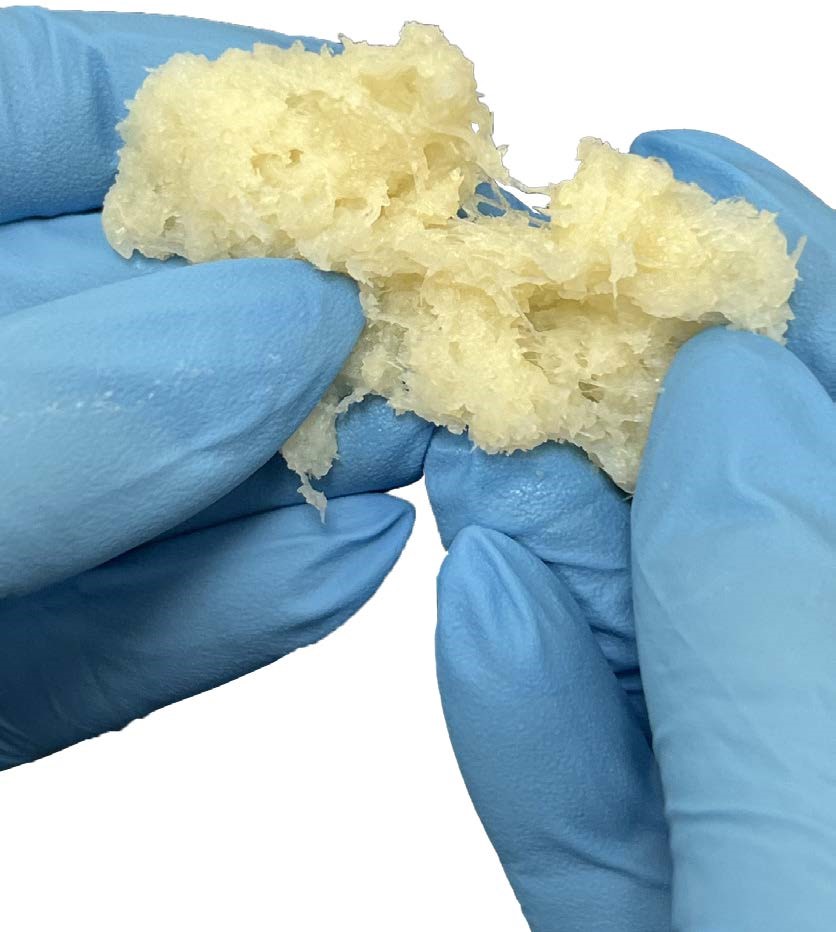
Sean Browne, President and CEO of Xtant Medical, stated, "New product launches are a key component of our strategy to drive margin improvement on higher sales and generate positive operating cash flow. OsteoVive Plus, which is manufactured in our Belgrade facility, expands our market opportunities and reinforces our position as an innovative medical technology company."
Mark Schallenberger, COO of Xtant Medical added, "OsteoVive Plus represents the latest advancement in cellular bone technology. Our new viable bone matrix features our Advanced Purloc™ Fiber Technology which includes a high concentration of elongated cortical fibers with consistent cross-sectional geometry, creating a moldable and cohesive allograft for optimal surgical placement."
OsteoVive Plus is manufactured at Xtant's state-of-the-art center for biologics processing in Belgrade, Montana.
The company will attend and showcase OsteoVive Plus along with its other products at the North American Spine Society (NASS) 2024 annual meeting.The conference is being held September 25-28, 2024 at McCormack Place in Chicago. Visit booth #4402 in the Exhibit Hall to learn more about Xtant and its product portfolio.
For additional information about OsteoVive Plus, please visit https://xtantmedical.com/product/osteovive-plus/ or contact sales support at cs@xtantmedical.com or (888) 886-9354.
About Xtant Medical Holdings, Inc.
Xtant Medical's mission of honoring the gift of donation so that our patients can live as full and complete a life as possible, is the driving force behind our company. Xtant Medical Holdings, Inc. (www.xtantmedical.com) is a global medical technology company focused on the design, development, and commercialization of a comprehensive portfolio of orthobiologics and spinal implant systems to facilitate spinal fusion in complex spine, deformity and degenerative procedures. Xtant people are dedicated and talented, operating with the highest integrity to serve our customers.
The symbols ™ and ® denote trademarks and registered trademarks of Xtant Medical Holdings, Inc. or its affiliates, registered as indicated in the United States, and in other countries. All other trademarks and trade names referred to in this release are the property of their respective owners.
Cautionary Statement Regarding Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements include statements that are predictive in nature, that depend upon or refer to future events or conditions, or that include words such as "intends," "expects," "anticipates," "plans," "believes," "estimates," "continue," "future," "will," "potential," "going forward," similar expressions or the negative thereof, and the use of future dates. Forward-looking statements in this release include the Company's expectations regarding the private placement, the timing for closing and the anticipated gross proceeds and use of net proceeds from the private placement. The Company cautions that its forward-looking statements by their nature involve risks and uncertainties, and actual results may differ materially depending on a variety of important factors, including, among others: risks and uncertainties surrounding the private placement; the Company's future operating results and financial performance; its ability to increase or maintain revenue; the Company's ability to become operationally self-sustaining and less reliant on third-party manufacturers and suppliers; risks associated with its acquisitions and the integration of those businesses; the Company's ability to implement successfully its future growth initiatives and risks associated therewith; possible future impairment charges to long-lived assets and goodwill and write-downs of excess inventory; the ability to remain competitive; the ability to innovate, develop and introduce new products and the success of those products; the ability to engage and retain new and existing independent distributors and agents and qualified personnel and the Company's dependence on key independent agents for a significant portion of its revenue; the effect of labor and hospital staffing shortages on the Company's business, operating results and financial condition, especially when they affect key markets; the effect of inflation, increased interest rates and other recessionary factors and supply chain disruptions; the effect of product sales mix changes on the Company's financial results; government and third-party coverage and reimbursement for Company products; the ability to obtain and maintain regulatory approvals and comply with government regulations; the effect of product liability claims and other litigation to which the Company may be subject; the effect of product recalls and defects; the ability to obtain and protect Company intellectual property and proprietary rights and operate without infringing the rights of others; risks associated with the Company's clinical trials; international risks; the ability to service Company debt, comply with its debt covenants and access additional indebtedness; the ability to obtain additional financing on favorable terms or at all; and other factors. Additional risk factors are contained in the Company's Annual Report on Form 10-K for the year ended December 31, 2023 filed with the SEC on April 1, 2024 and subsequent SEC filings by the Company. Investors are encouraged to read the Company's filings with the SEC, available at www.sec.gov, for a discussion of these and other risks and uncertainties. The Company undertakes no obligation to release publicly any revisions to any forward-looking statements to reflect events or circumstances after the date hereof or to reflect the occurrence of unanticipated events, except as required by law. All forward-looking statements attributable to the Company or persons acting on its behalf are expressly qualified in their entirety by this cautionary statement.
Investor Relations Contact
Brett Maas
Managing Partner, Hayden IR
brett@haydenir.com
(646) 536-7331
SOURCE: Xtant Medical Holdings, Inc.
View the original press release on accesswire.com







