XBiotech Results from Randomized Double-Blinded Phase 1/2 Study Suggest Potential Breakthrough Treatment for Advanced Pancreatic Cancer
Rhea-AI Summary
XBiotech (NASDAQ: XBIT) announced promising results from its Phase 1/2 trial for advanced pancreatic cancer treatment using Natrunix in combination with a standard chemotherapy regimen. The study, titled 1-BETTER, enrolled 65 subjects who received either the Natrunix combination or a placebo. Key findings include a 28% reduction in significant adverse events in the Natrunix group, a 33% decrease in hospitalization days, and a notable improvement in patient-reported outcomes such as reduced fatigue (22%) and pain (41%). While overall survival rates showed a trend favoring Natrunix, the small sample size led to a borderline statistical significance (p = 0.096). XBiotech emphasizes the potential of Natrunix to improve tolerability and efficacy of pancreatic cancer treatment.
Positive
- 28% reduction in significant adverse events for Natrunix group.
- 33% decrease in hospitalization days for Natrunix group (80 days vs. 120 days).
- 22% reduction in fatigue and 41% reduction in pain in Natrunix group.
- No dose-limiting toxicities observed in Phase 1 study.
- Trend towards prolonged survival in Natrunix group with 8 surviving past 330 days.
Negative
- Borderline statistical significance in overall survival data (p = 0.096).
- Small sample size reduces the certainty of the findings.
News Market Reaction
On the day this news was published, XBIT declined 13.49%, reflecting a significant negative market reaction.
Data tracked by StockTitan Argus on the day of publication.
Findings Show Trends for Reduced Toxicities and Better Outcomes for Subjects Receiving ONIVYDE/5-FU Combination and Targeted anti-IL-1alpha Therapy
AUSTIN, Texas, June 18, 2024 (GLOBE NEWSWIRE) -- XBiotech (NASDAQ: XBIT) announced today data from its Phase 1/Phase 2 randomized, double-blind, placebo-controlled multi-center study for advanced pancreatic cancer. Known as 1-BETTER, the study examined Natrunix (anti-interleukin-1alpha) antibody in combination with an established chemotherapy regimen (ONIVYDE (ON) + 5-Fluorouracil (5FU) + Leucovorin (LV), a regimen that is already widely used for treating pancreatic cancer but is associated with difficult toxicities and less then ideal survival outcomes. Natrunix was being evaluated as an anti-cancer agent for use in cytotoxic chemotherapy combinations where the Company believes it might potentially also improve tolerability of the chemotherapy.
The Phase 1 portion was a dose escalation study in metastatic pancreatic cancer patients to determine if dose limiting toxicities (DLTs) occurred in combination with the ON+5FU+LV regimen in second- or third-line setting. DLTs were not expected with Natrunix and none were seen. The Natrunix dose in the Phase 2 portion was thus the highest dose used in the Phase 1 portion.
Sixty-five subjects were randomized into the Phase 2 study on a 1:1 basis to receive either Natrunix+ ON+5FU+LV (Arm1) or Placebo +ON+5FU+LV (Arm2). There were 33 subjects enrolled into Arm1 and 32 into Arm2. The Phase 2 treatment period was 24-weeks with subjects receiving therapy once every other week for a total of 12 cycles.
Subjects included in the study had confirmed metastatic, unresectable, or recurrent pancreatic adenocarcinoma of exocrine pancreas and were required to have had disease progression after one prior gemcitabine-based therapy or one FOLFIRINOX and gemcitabine containing therapy. All patients were required to have at least one measurable lesion according to Response Evaluation Criteria in Solid Tumor (RECIST v1.1).
The primary endpoint for the Phase 2 study was to assess the safety and tolerability of Natrunix when used with the ON+5FU+LV combination. Overall, there were fewer adverse events (AEs) of any kind during the 24-week treatment period for the Natrunix arm compared to placebo (297 vs 336), with markedly fewer events in specific categories of adverse events during that time. There was a
Subjects receiving the Natrunix combination also reported a
Severe diarrhea that can be life -threatening is a significant complication for the ON+5FU+LV regimen. There was a two-fold reduction (
Overall Survival (OS), one of the secondary endpoints for the Phase 2 study, was conventionally defined in as time from randomization to death. The sample size for the study included intent-to-treat analysis of 33 subjects randomized into the Natrunix + ON+5FU+LV arm versus 32 subjects in Placebo + ON+5FU+LV arm. A Kaplan-Meier Survival Curve using a product limit comparison method was performed. This data highlights the observation that no subjects in the placebo ON+5FU+LV group (n=32) survived for longer than 330 days, whereas 8 subjects in the Natrunix ON+5FU+LV arm (n=33) were still alive as of day 330. Considering the small sample size, the borderline statistically significant p-value of p = 0.096 suggests prolonged survival for subjects receiving the Natrunix regimen.
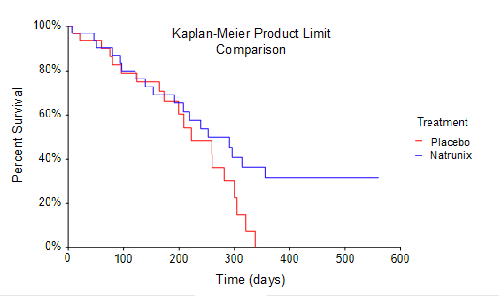
The lead investigator for the study, David J. Park, MD Medical Oncologist, Medical Director for the providence St. Jude Crosson Institute, Fullerton, CA stated “Treatment of advanced pancreatic cancer in the second and third line settings presents significant challenges in terms of toxicity as well as efficacy. To observe these trends for reduced toxicity and potential survival benefit is remarkable, particularly given the limited sample size. The potential interaction between reduced toxicity, more time on treatment and improvement in survival makes intuitive sense for clinicians who treat these patients. These findings are extremely important.”
While there was a relatively small number of pancreatic cancer patients enrolled in the Phase 2 portion of the study, in the Company’s opinion, the findings show better outcomes for the Natrunix + ON+5FU+LV group as compared to the control arm. The Company believes that the reduced number of serious and adverse events, the significant reduction in hospitalization, and improved OS during the respective time periods described above for each of these metrics suggest that Natrunix could represent a breakthrough advance for the treatment of pancreatic cancer.
About XBiotech
XBiotech is pioneering the discovery and development of targeted antibodies based on its True Human™ technology. The company’s mission is to rethink the way antibody medicines are discovered and commercialized by advancing its robust pipeline of truly natural human antibodies for treating serious diseases such as inflammatory conditions like rheumatology, infectious disease, cardiovascular disease and cancer. XBiotech has several candidate products including Natrunix. Cloned from individual donors who possess natural immunity against certain targeted diseases, XBiotech’s pipeline of True Human antibodies are intended to deliver unmatched safety and efficacy. Located just minutes from downtown Austin, the XBiotech campus headquarters includes GMP manufacturing facilities, research and testing laboratories, infectious disease research facilities, and quality control and clinical operations. For more information, visit www.xbiotech.com.
Cautionary Note on Forward-Looking Statements and Study Results
This press release contains forward-looking statements, including declarations regarding management's beliefs and expectations that involve substantial risks and uncertainties. Forward-looking statements are subject to inherent risks and uncertainties in predicting future results and conditions that could cause the actual results to differ materially from those projected in these forward-looking statements. These risks and uncertainties are subject to the disclosures set forth in the "Risk Factors" section of certain of our SEC filings. Any forward-looking statements that we make in this press release speak only as of the date of this press release. We assume no obligation to update our forward-looking statements whether as a result of new information, future events or otherwise, after the date of this press release. The Company makes no representations regarding OS or any other metric beyond the time periods specifically discussed herein. There can be no assurance that any study results discussed in this press release will be replicated in future studies or that Natrunix will be approved by the Food and Drug Administration or any other regulator.
Contact
Wenyi Wei
wwei@xbiotech.com
Tel. 737-207-4600
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/7a17b3d3-b304-47ae-b1e7-0950f0301f12










