Beyond Cancer Presents Promising Preclinical Data on Low Volume Ultra-High Concentration Nitric Oxide (LV UNO) Therapy at the 2024 Society for Immunotherapy of Cancer (SITC) Annual Meeting
Beyond Cancer presented promising preclinical data on Low Volume Ultra-High Concentration Nitric Oxide (LV UNO) therapy at SITC 2024. The study showed that LV UNO combined with anti-rPD-L1 doubled tumor growth inhibition and improved survival in MAT B III tumor-bearing rats compared to anti-rPD-L1 alone. Additionally, LV UNO proved more effective than High Volume UNO in reducing tumor volumes in CT26 BALB/c mice when combined with anti-mPD-1 treatment. The research demonstrated enhanced nitro-tyrosine staining with LV UNO, indicating better tumor distribution of nitric oxide and potential improved safety outcomes.
Beyond Cancer ha presentato dati preclinici promettenti sulla terapia a Basso Volume e Ultra-Alta Concentrazione di Monossido di Azoto (LV UNO) al SITC 2024. Lo studio ha dimostrato che LV UNO combinato con anti-rPD-L1 ha raddoppiato l'inibizione della crescita tumorale e ha migliorato la sopravvivenza nei ratti portatori di tumore MAT B III rispetto all'anti-rPD-L1 da solo. Inoltre, LV UNO si è dimostrato più efficace del High Volume UNO nella riduzione dei volumi tumorali nei topi CT26 BALB/c quando combinato con il trattamento anti-mPD-1. La ricerca ha evidenziato una maggiore colorazione della nitro-tirosina con LV UNO, indicando una migliore distribuzione del monossido di azoto nel tumore e potenziali risultati di sicurezza migliorati.
Beyond Cancer presentó datos preclínicos prometedores sobre la terapia de Bajo Volumen y Ultra-Alta Concentración de Óxido Nítrico (LV UNO) en el SITC 2024. El estudio mostró que LV UNO combinado con anti-rPD-L1 duplicó la inhibición del crecimiento tumoral y mejoró la supervivencia en ratas con tumor MAT B III en comparación con solo anti-rPD-L1. Además, LV UNO demostró ser más efectivo que el Alto Volumen UNO para reducir volúmenes tumorales en ratones CT26 BALB/c cuando se combina con el tratamiento anti-mPD-1. La investigación mostró un aumento en la tinción de nitro-tirosina con LV UNO, lo que indica una mejor distribución tumoral de óxido nítrico y posibles resultados de seguridad mejorados.
비욘드 캔서는 SITC 2024에서 저용량 초고농축 질산화물(LV UNO) 치료에 대한 유망한 전임상 데이터를 발표했습니다. 연구 결과 LV UNO와 항-rPD-L1의 조합은 종양 성장 억제를 두 배로 증가시켰으며 MAT B III 종양을 가진 쥐에서 항-rPD-L1 단독 사용보다 생존율을 개선했습니다. 또한, LV UNO는 항-mPD-1 치료와 결합 시 고용량 UNO보다 종양 부피 감소에 더 효과적임을 입증했습니다. 연구는 LV UNO와 함께 질산화물의 더 나은 종양 분포를 나타내는 질소-티로신 염색이 개선된 것을 보여주었으며, 잠재적으로 안전성 결과가 개선될 수 있음을 시사합니다.
Beyond Cancer a présenté des données précliniques prometteuses sur la thérapie à faible volume et ultra-haute concentration de monoxyde d'azote (LV UNO) au SITC 2024. L'étude a montré que LV UNO associé à l'anti-rPD-L1 a doublé l'inhibition de la croissance tumorale et amélioré la survie chez les rats porteurs de tumeurs MAT B III par rapport à l'anti-rPD-L1 seul. De plus, LV UNO s'est révélé plus efficace que le High Volume UNO pour réduire les volumes tumoraux chez les souris CT26 BALB/c lorsqu'il était combiné avec un traitement anti-mPD-1. La recherche a démontré une coloration nitro-tyrosine améliorée avec LV UNO, indiquant une meilleure distribution tumorale du monoxyde d'azote et des résultats de sécurité potentiellement améliorés.
Beyond Cancer präsentierte vielversprechende präklinische Daten zur Therapie mit niedrigem Volumen und ultra-hoher Konzentration von Stickstoffmonoxid (LV UNO) auf dem SITC 2024. Die Studie zeigte, dass LV UNO in Kombination mit anti-rPD-L1 das Tumorwachstumshemmung verdoppelte und die Überlebensrate bei MAT B III Tumor tragenden Ratten im Vergleich zu anti-rPD-L1 allein verbesserte. Darüber hinaus bewies LV UNO eine höhere Effektivität als High Volume UNO bei der Reduktion von Tumorvolumina in CT26 BALB/c Mäusen, wenn es mit anti-mPD-1 Behandlung kombiniert wurde. Die Forschung zeigte eine erhöhte Nitro-Tyrosin-Färbung mit LV UNO, was auf eine bessere Tumorverteilung von Stickstoffmonoxid und potenziell verbesserte Sicherheitsauswirkungen hinweist.
- LV UNO + anti-rPD-L1 doubled tumor growth inhibition rate
- Demonstrated prolonged survival advantage by Day 37
- LV UNO showed superior efficacy compared to HV UNO in tumor volume reduction
- Enhanced nitro-tyrosine staining indicates improved tumor distribution
- None.
Insights
The preclinical data demonstrates significant potential for Beyond Cancer's Low Volume Ultra-High Concentration Nitric Oxide (LV UNO) therapy. Key findings show that when combined with anti-rPD-L1, LV UNO achieved doubled tumor growth inhibition and improved survival outcomes compared to standalone checkpoint inhibitor treatment. The 25,000 ppm and 100,000 ppm dosing levels showed promising results.
The improved nitro-tyrosine staining (Grade 3 vs Grade 1) indicates better tumor penetration with LV UNO compared to HV UNO, suggesting enhanced therapeutic efficacy. This could translate to better clinical outcomes while potentially reducing side effects due to the lower volume administration. The advancement to Phase 1b trials represents a significant milestone in validating this novel combination approach for solid tumors.
- LV UNO in combination with anti-rPD-L1 resulted in prolonged survival and doubled the tumor growth inhibition rate in MAT B III tumor bearing rats compared to anti-rPD-L1 alone
- LV UNO compares favorably to HV UNO administration in reducing tumor volumes in combination with anti-mPD-1 in CT26 BALB/c mice
- Increased nitro-tyrosine staining in LV UNO treated tumors indicates an improved distribution of UNO in the tumor leading to potentially improved outcomes and an enhanced safety profile
HAMILTON, Bermuda, Nov. 11, 2024 (GLOBE NEWSWIRE) -- Beyond Cancer, Ltd., a clinical stage biotechnology company developing ultra-high concentration nitric oxide (UNO) as an immunotherapeutic for solid tumors, today announced key data demonstrating the efficacy of Low Volume UNO (LV UNO, < 100 mL) when combined with immune checkpoint inhibitors, which were presented in two posters at the Society for Immunotherapy of Cancer (SITC) Annual Meeting 2024.
In a study using MAT B III tumor-bearing rats, LV UNO combined with anti-rPD-L1 doubled the tumor growth inhibition rate and led to improved survival outcomes compared to anti-rPD-L1 alone. These promising results support further clinical evaluation of LV UNO alongside checkpoint inhibitors for enhanced patient benefits and safety.
Additionally, LV UNO was shown to be more effective than High Volume UNO (HV UNO; 1 L) in reducing tumor volumes in CT26 BALB/c mice when paired with anti-mPD-1 treatment. Notably, LV UNO enhanced nitro-tyrosine staining, indicating better tumor distribution of nitric oxide and potentially improved safety outcomes.
Study Highlights:
Data presented in abstract #: 723, which is titled “Intratumoral Administration of Low Volume Ultra High Concentration Nitric Oxide and Anti-rPD-L1 Treatment Leads to Prolonged Survival in MAT B III Tumor-Bearing Rats,” demonstrated that doses of LV UNO at 25,000 ppm or 100,000 ppm combined with anti-rPD-L1 at 10 mg/kg led to a reduction in tumor volume as compared to anti-rPD-L1 only treated MAT B III bearing Fischer rats when dosed every third day for a total of six doses. Importantly, by Day 37 a prolonged survival advantage was observed in the LV UNO combination arm.
Dr. Jedidiah Monson, Chief Medical Officer of Beyond Cancer, commented, “Evaluating UNO alongside anti-rPD-L1 in MAT B III tumor bearing rats showed data that align with our previously published results in mice. As a result, we are excited to advance this program and evaluate LV UNO administration combined with checkpoint inhibitors in the clinic. We believe this approach could offer meaningful benefits for patients with the potential of an improved safety profile.”
LV UNO and Anti-rPD-L1 Doubled Survival by Day 37 versus Anti-rPD-L1 Alone
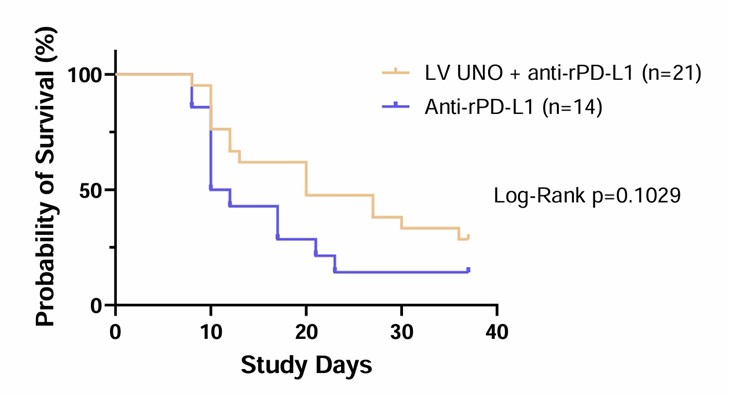
Caption: Anti-rPD-L1 mAb in combination with either 25,000 ppm or 100,000 ppm UNO resulted in prolonged survival
Data presented in abstract #: 724, which is titled “Intratumoral Administration of Low Volume Ultra-High Concentration Nitric Oxide and Immune Checkpoint Inhibitors in CT26 Tumor-Bearing Mice,” showed a greater reduction in primary tumor volume in mice receiving LV UNO in combination with anti-mPD-1 (n=25) at Day 11, compared to mice receiving HV UNO administration with anti-mPD-1 (n=14). Both combination treatment arms (HV UNO and LV UNO with anti-mPD-1) resulted in better tumor reduction than anti-mPD-1 alone (n=16). The enhanced effectiveness of the LV UNO administration method may be attributed, in part, to the improved distribution of UNO in the tumor, as measured by nitro-tyrosine immunohistochemistry staining following treatment. Nitro-tyrosine is a post translational modification product resulting from the reaction of tyrosine with reactive nitrogen species. HV UNO administration revealed Grade 1 staining, defined as a few positive cells [< 5 cells] versus Grade 3 staining, defined as a mild reaction [15–25 cells] for LV UNO administration (p < 0.01 compared to sham, ANOVA and Tukey HSD statistical methods). Tumors treated with sham or nitrogen showed the lowest levels of nitro-tyrosine staining.
Effect of LV UNO in Combination with Anti-mPD-1 mAb Reduced Primary Tumor Volume and Compares Favorably to HV UNO
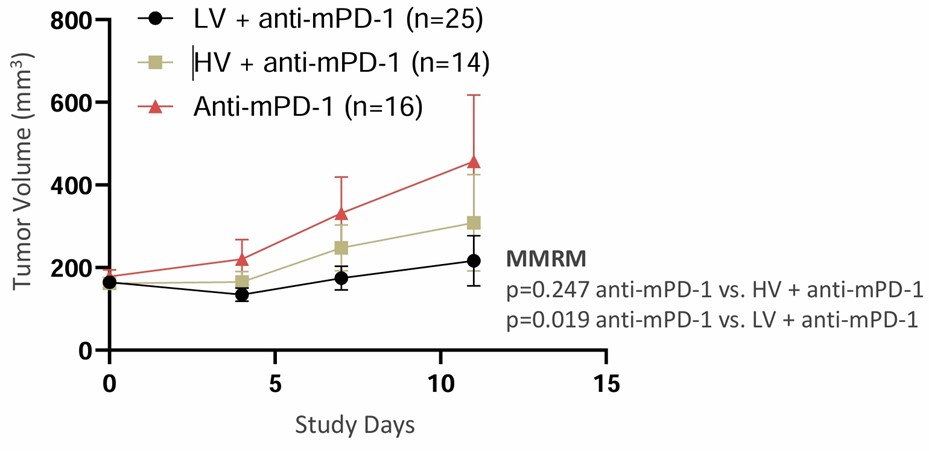
Caption: LV UNO in Combination with Anti-mPD-1 mAb Reduced Primary CT26 Tumor Volume
“These preclinical results prompted our decision to commence the Phase 1b study to investigate the combination of LV UNO with immune checkpoint inhibitors. The clinical study aims to evaluate the potential of UNO to enhance the type, density, and distribution of immune cells within the tumor microenvironment, particularly in cases of PD-1 progression and prolonged stable disease,” stated Dr. Selena Chaisson, Chief Executive Officer, and Director of Beyond Cancer.
A copy of the ePublications can be accessed on the Science and Technology page of the Company’s website.
About Nitric Oxide
Nitric Oxide (NO) is a potent molecule, naturally synthesized in the human body, proven to play a critical role in a broad array of biological functions. In the airways, NO targets the vascular smooth muscle cells that surround the small resistance arteries in the lungs. Currently, exogenous inhaled NO is used in adult respiratory distress syndrome, post certain cardiac surgeries and persistent pulmonary hypertension of the newborn to treat hypoxemia. Additionally, NO is believed to play a key role in the innate immune system and in vitro studies suggest that NO possesses anti-microbial activity not only against common bacteria, including both gram-positive and gram-negative, but also against other diverse pathogens.
About UNO Therapy for Solid Tumors
Cancer is the second leading cause of death globally, with tumor metastases responsible for approximately
About Beyond Cancer, Ltd.
Beyond Cancer, Ltd. is a development-stage biopharmaceutical and medical device company utilizing ultra-high concentration nitric oxide (UNO) via a proprietary delivery platform to treat primary tumors and prevent metastatic disease. Nitric oxide at ultra-high concentrations has been reported to show anticancer properties and to potentially serve as a chemosensitizer and radiotherapy enhancer. A first-in-human study is underway in patients with solid tumors. Beyond Cancer is also conducting preclinical studies of UNO in multiple solid tumor models to inform additional treatment protocols. For more information, visit www.beyondcancer.com.
Forward Looking Statements
This press release contains “forward-looking statements” concerning the potential safety and efficacy of inhaled nitric oxide and the ultra-high concentration nitric oxide product candidate, as well as its therapeutic potential in a number of indications; and the potential impact on patients and anticipated benefits associated with inhaled nitric oxide and the ultra-high concentration nitric oxide product candidate. Forward-looking statements include statements about expectations, beliefs, or intentions regarding product offerings, business, results of operations, strategies or prospects. You can identify such forward-looking statements by the words “expects,” “plans,” “anticipates,” “believes” “expects,” “intends,” “looks forward,” “projects,” “goal,” “assumes,” “targets” and similar expressions and/or the use of future tense or conditional constructions (such as “will,” “may,” “could,” “should” and the like) and by the fact that these statements do not relate strictly to historical or current matters. Rather, forward-looking statements relate to anticipated or expected events, activities, trends or results as of the date they are made. Because forward-looking statements relate to matters that have not yet occurred, these statements are inherently subject to risks and uncertainties that could cause actual results to differ materially from any future results expressed or implied by the forward-looking statements. These forward-looking statements are only predictions and reflect views as of the date they are made with respect to future events and financial performance. Many factors could cause actual activities or results to differ materially from the activities and results anticipated in forward-looking statements, including risks related to the ability to raise additional capital; the timing and results of future pre-clinical studies and clinical trials concerning the ultra-high concentration nitric oxide product candidate; the potential that regulatory authorities, including the FDA and comparable non-U.S. regulatory authorities, may not grant or may delay approval for the ultra-high concentration nitric oxide product candidate; the approach to discover and develop novel drugs, which is unproven and may never lead to efficacious or marketable products; obtaining, maintaining and protecting intellectual property utilized by products; competition from others using similar technology and others developing products for similar uses; dependence on collaborators; and other risks, which may, in part, be identified and described in the “Risk Factors” section of Beyond Air, Inc.’s most recent Annual Report on Form 10-K and other of its filings with the Securities and Exchange Commission, all of which are available on Beyond Air, Inc.’s website. Beyond Cancer and Beyond Air undertake no obligation to update, and have no policy of updating or revising, these forward-looking statements, except as required by applicable law.
CONTACTS:
Corey Davis, PhD
LifeSci Advisors, LLC
Cdavis@lifesciadvisors.com
(212) 915-2577
Matt Johnson, Head of Corporate Development & Strategy
Beyond Cancer, Ltd.
Mjohnson@beyondcancer.com
Photos accompanying this announcement are available at:
https://www.globenewswire.com/NewsRoom/AttachmentNg/e845a3df-0d68-42e7-9a2a-1cb8e129bf5b
https://www.globenewswire.com/NewsRoom/AttachmentNg/798962a0-302e-4bc1-b063-b10b180fdc2a








