WuXi Biologics Successfully Completes First Scale-Up of High-Productivity Bioprocessing Platform WuXiUI™ in 2,000L GMP Manufacturing
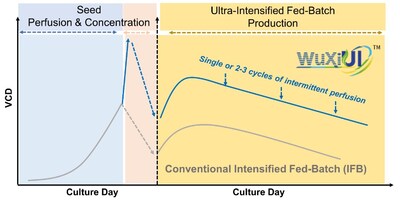
WuXi Biologics (WXXWY) has successfully completed its first scale-up to 2,000L drug substance (DS) GMP manufacturing using its proprietary WuXiUI™ ultra-intensified fed-batch platform. The platform achieved a 4-fold productivity improvement compared to traditional fed-batch processes, with a titer of 18 g/L. This advancement, combined with enhanced downstream technology, resulted in a 50% reduction in downstream processing time and a final DS yield of 70%. The improvements led to significant reductions in manufacturing COGS and waste generation. The success of WuXiUI™, launched in 2023, demonstrates its readiness for larger-scale production and its potential to meet growing demand for therapeutic proteins and antibodies with lower costs and a reduced carbon footprint.
WuXi Biologics (WXXWY) ha completato con successo il suo primo ampliamento alla produzione di principi attivi (DS) GMP a 2.000L utilizzando la sua piattaforma proprietaria WuXiUI™ ultra-intensified fed-batch. La piattaforma ha raggiunto un incremento della produttività di 4 volte rispetto ai processi tradizionali di fed-batch, con un titer di 18 g/L. Questo progresso, insieme a una tecnologia di downstream migliorata, ha portato a una riduzione del 50% del tempo di elaborazione downstream e a un rendimento finale di DS del 70%. I miglioramenti hanno comportato significative riduzioni nei costi di produzione (COGS) e nella generazione di rifiuti. Il successo di WuXiUI™, lanciato nel 2023, dimostra la sua prontezza per la produzione su larga scala e il suo potenziale per soddisfare la crescente domanda di proteine terapeutiche e anticorpi con costi inferiore e una riduzione dell'impronta di carbonio.
WuXi Biologics (WXXWY) ha completado con éxito su primera ampliación a la fabricación de sustancia activa (DS) GMP a escala de 2.000L utilizando su plataforma propietaria WuXiUI™ ultra-intensified fed-batch. La plataforma logró una mejora de productividad de 4 veces en comparación con los procesos tradicionales de fed-batch, con un título de 18 g/L. Este avance, combinado con una tecnología de downstream mejorada, resultó en una reducción del 50% en el tiempo de procesamiento downstream y un rendimiento final de DS del 70%. Las mejoras condujeron a reducciones significativas en los costos de fabricación (COGS) y en la generación de residuos. El éxito de WuXiUI™, lanzado en 2023, demuestra su preparación para la producción a gran escala y su potencial para satisfacer la creciente demanda de proteínas terapéuticas y anticuerpos con costos más bajos y una huella de carbono reducida.
우시 생명과학(WXXWY)은 자사 고유의 WuXiUI™ 초집중 배치 플랫폼을 사용하여 2,000L 약물 물질(DS) GMP 제조의 첫 번째 스케일업을 성공적으로 완료했습니다. 이 플랫폼은 전통적인 배치 프로세스에 비해 4배의 생산성 향상을 달성했으며, 타이터는 18 g/L입니다. 이 발전은 향상된 다운스트림 기술과 결합되어 다운스트림 가공 시간 50% 감소와 최종 DS 수율 70%를 가져왔습니다. 이러한 개선은 제조 COGS 및 폐기물 발생을 크게 줄였습니다. 2023년에 출시된 WuXiUI™의 성공은 대규모 생산을 위한 준비가 되었음을 보여주며, 더 낮은 비용과 줄어든 탄소 발자국으로 치료 단백질 및 항체에 대한 증가하는 수요를 충족할 수 있는 잠재력을 지니고 있습니다.
WuXi Biologics (WXXWY) a réussi à terminer son premier passage à une production de substance active (DS) GMP à l'échelle de 2.000L en utilisant sa plateforme propriétaire WuXiUI™ ultra-intensified fed-batch. Cette plateforme a réalisé une amélioration de la productivité de 4 fois par rapport aux procédés traditionnels en batch, avec un titre de 18 g/L. Cette avancée, combinée à une technologie de downstream améliorée, a entraîné une réduction de 50% du temps de traitement downstream et un rendement final de DS de 70%. Les améliorations ont conduit à des réductions significatives des coûts de fabrication (COGS) et de la génération de déchets. Le succès de WuXiUI™, lancé en 2023, démontre sa préparation à une production à grande échelle et son potentiel à répondre à la demande croissante de protéines thérapeutiques et d'anticorps à coûts réduits et avec une empreinte carbone moindre.
WuXi Biologics (WXXWY) hat erfolgreich die erste Skalierung auf 2.000L Arzneistoff (DS) GMP-Herstellung mit seiner proprietären WuXiUI™ ultra-intensified fed-batch Plattform abgeschlossen. Die Plattform erzielte eine 4-fache Produktivitätssteigerung im Vergleich zu traditionellen fed-batch-Prozessen, mit einem Titer von 18 g/L. Dieser Fortschritt, verbunden mit verbesserter Downstream-Technologie, führte zu einer Reduzierung der Downstream-Bearbeitungszeit um 50% und einer finalen DS-Ausbeute von 70%. Die Verbesserungen führten zu erheblichen Einsparungen bei den Herstellungskosten (COGS) und der Abfallgeneration. Der Erfolg von WuXiUI™, das 2023 eingeführt wurde, zeigt, dass es bereit für die Produktion im größeren Maßstab ist und das Potenzial hat, die wachsende Nachfrage nach therapeutischen Proteinen und Antikörpern zu geringeren Kosten und mit einem reduzierten Kohlenstoff-Fußabdruck zu decken.
- Achieved 4-fold productivity improvement in drug substance manufacturing
- 50% reduction in downstream processing time
- 70% final drug substance yield
- Significant reduction in manufacturing COGS
- 30-50% reduction in materials and consumables utilization
- Lower carbon footprint due to efficient media consumption and reduced waste generation
- None.
- By leveraging its ultra-intensified fed-batch platform, WuXiUI™, WuXi Biologics has completed its first scale-up to 2,000L drug substance (DS) GMP manufacturing, achieving a 4-fold productivity improvement compared to the traditional fed-batch process
- The competitive performance achieved through the utilization of both WuXiUI™ and the proprietary platform cell culture media MagniCHO™ led to significant reduction in overall DS manufacturing COGS
- The consistent performance of WuXiUI™ – from small scales to 2,000L GMP manufacturing – is a testament to the advancement of the technology as a mature and robust platform capable of significantly improving the cost-effectiveness of biologics production
In addition, the company's enhanced downstream technology platform enabled doubled purification processing capacity and similar impurity removal, which resulted in a
The WuXiUI™ platform, launched in 2023, enhances the productivity of multiple different CHO or other mammalian cell lines that express diverse product modalities, while maintaining desirable product qualities. It provides an effective solution for global clients to meet the growing demand for therapeutic proteins and antibodies with lower COGS. At the same time, it allows for a lower carbon footprint due to its more efficient media consumption, lower waste generation, and reduced demand for building space in the production line. The successful scale-up of the WuXiUI™ platform from bench scales to 2,000L GMP manufacturing confirms the robustness of the technology and its readiness for larger-scale production.
WuXiUI™'s strong performance was boosted through the application of MagniCHO™, WuXi Biologics' proprietary platform cell culture media enriched with nutrients for intensified processes. Furthermore, operation efficiency and production robustness were improved by integrating the Raman Process Analytical Technology (PAT) tool into the scale-up manufacturing, not only for process monitoring, but also – for the first time under GMP settings – to provide real-time automated process control.
Dr. Chris Chen, CEO of WuXi Biologics, commented, "The successful application and achievement of WuXiUI™ is a result of our relentless pursuit of technology innovation to speed up biologics development while achieving cost efficiency for global clients. With this milestone, we have enhanced our capabilities to enable clients to bring more affordable, high-quality biologics to market, benefiting patients worldwide."
About WuXi Biologics
WuXi Biologics (stock code: 2269.HK) is a leading global Contract Research, Development and Manufacturing Organization (CRDMO) offering end-to-end solutions that enable partners to discover, develop and manufacture biologics – from concept to commercialization – for the benefit of patients worldwide.
With over 12,000 skilled employees in
WuXi Biologics views Environmental, Social, and Governance (ESG) responsibilities as an integral component of our ethos and business strategy, and we aim to become an ESG leader in the biologics CRDMO sector. Our facilities use next-generation biomanufacturing technologies and clean-energy sources. We have also established an ESG committee led by our CEO to steer the comprehensive ESG strategy and its implementation, enhancing our commitment to sustainability.
For more information about WuXi Biologics, please visit: www.wuxibiologics.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/wuxi-biologics-successfully-completes-first-scale-up-of-high-productivity-bioprocessing-platform-wuxiui-in-2-000l-gmp-manufacturing-302234885.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/wuxi-biologics-successfully-completes-first-scale-up-of-high-productivity-bioprocessing-platform-wuxiui-in-2-000l-gmp-manufacturing-302234885.html
SOURCE WuXi Biologics