Viatris Reports Strong Financial and Operational Results for the Third Quarter 2023 and Reaffirms Full-Year 2023 Adjusted EBITDA and Free Cash Flow Guidance Ranges[1]
- Viatris Inc. reports strong financial results for the third quarter of 2023, with total revenues of $3.94 billion and adjusted EBITDA of $1.36 billion. The company also signals the continuation of its growth journey with the second consecutive quarter of year-over-year operational revenue growth on a divestiture-adjusted basis. The decision to revise full-year total revenues guidance range solely to reflect the expected negative impact of foreign exchange demonstrates transparency and adaptability to market conditions. Additionally, the commitment to complete all planned divestitures by the end of the first half of 2024 shows a clear strategic focus on streamlining operations and maximizing efficiency. The declaration of a quarterly dividend of $0.12 per share indicates the company's confidence in its financial position and commitment to returning value to shareholders.
- None.
Insights
Analyzing...
- Reports Total Revenues of
$3.94 Billion U.S. GAAP Net Earnings of$332 Million $1.36 Billion U.S. GAAP Net Cash Provided by Operating Activities of$834 Million $738 Million - Strong Results Signal Continuation of Growth Journey with Second Consecutive Quarter of Year-over-Year Operational Revenue Growth on a Divestiture-Adjusted Basis[2]
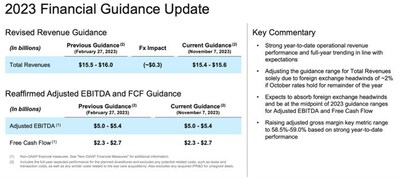
- Revises Full-Year Total Revenues Guidance Range Solely to Reflect the Expected Negative Impact of Foreign Exchange
- On Track to Complete All Planned Divestitures by the End of the First Half of 2024[3]
- Board of Directors Declares Quarterly Dividend of
$0.12
Executive Commentary
Viatris CEO Scott A. Smith said: "Viatris had another outstanding quarter in Q3. It is our second consecutive quarter of year-over-year operational revenue growth on a divestiture-adjusted basis and our eleventh straight quarter of strong operational results. These results are an indication of the continuing momentum we are building as we prepare to bring Phase 1 of our strategic plan to successful completion. Operationally, we are continuing to see strong performance globally across our businesses. We are on track to complete all our planned divestitures by the end of the first half of 2024. We are now shifting our focus to Phase 2 and to adding to the strength of our stable base by building the business in areas with the greatest potential for growth, patient impact and shareholder value."
Viatris President Rajiv Malik said: "We achieved yet another strong quarter of commercial execution around the globe. As a result of our continued advancement of our robust and deep pipeline, we are on track to deliver
Viatris CFO Sanjeev Narula said: "The diversity of our global portfolio and platforms continue to drive strong gross margins. Our solid and continued durable free cash flow generation has served to further strengthen our balance sheet while returning significant capital to shareholders. Based on the underlying fundamentals of our business, we believe we are well positioned to deliver on our financial guidance for the remainder of 2023 and for a strong start to 2024."
[1] Viatris is not providing forward-looking guidance for |
[2] For the three months ended September 30, 2023, total net sales declined |
[3] Divestitures are subject to regulatory approvals, completion of any consultations with employee representatives (where applicable), receipt of required consents and other closing conditions, including, in the case of the API business divestiture, a financing condition. |
2023 Financial Guidance
The Company is not providing forward-looking guidance for
Return of Capital to Shareholders
Viatris announced that, on November 6, 2023, its Board of Directors declared a quarterly dividend of
Viatris paid a quarterly cash dividend of
Conference Call and Earnings Materials
Viatris Inc. will host a conference call and live webcast, today at 5:00 p.m. ET, to discuss the Company's financial results for the third quarter of 2023.
Investors and the general public are invited to listen to a live webcast of the call at investor.viatris.com or by calling 800.274.8461 or 203.518.9783 for international callers (Conference ID: VTRSQ323). The "Viatris Q3 Earnings Presentation," which will be referenced during the call, can be found at investor.viatris.com. A replay of the webcast also will be available on the website.
Financial Summary | |||||||||
Three Months Ended | |||||||||
September 30, | |||||||||
(Unaudited; in millions, except %s) | 2023 | 2022 | Reported | Operational | Divestiture- | ||||
Total Net Sales | (3) % | (3) % | 1 % | ||||||
Developed Markets | 2,408.5 | 2,431.5 | (1) % | (4) % | 2 % | ||||
Emerging Markets | 642.5 | 678.9 | (5) % | — % | 2 % | ||||
JANZ | 334.5 | 383.0 | (13) % | (8) % | (6) % | ||||
548.4 | 574.0 | (4) % | — % | — % | |||||
Net Sales by Product Category | |||||||||
Brands | — % | (1) % | (1) % | ||||||
Complex Gx | 174.4 | 320.2 | (46) % | (46) % | 25 % | ||||
Generics | 1,226.4 | 1,206.9 | 2 % | 3 % | 3 % | ||||
(3) % | |||||||||
42.9 % | 42.9 % | ||||||||
Adjusted Gross Profit (3) | (5) % | ||||||||
Adjusted Gross Margin (3) | 59.2 % | 60.5 % | |||||||
$ 331.6 | $ 354.3 | (6) % | |||||||
Adjusted Net Earnings (3) | $ 952.8 | (10) % | |||||||
EBITDA (3) | (4) % | ||||||||
Adjusted EBITDA (3) | (9) % | (9) % | (6) % | ||||||
$ 834.1 | $ 869.0 | (4) % | |||||||
Capital expenditures | 95.9 | 103.9 | (8) % | ||||||
Free cash flow (3) (4) | $ 738.2 | $ 765.1 | (4) % | ||||||
___________ | |
(1) | Represents operational change for net sales and adjusted EBITDA which excludes the impacts of foreign currency translation. See "Certain Key Terms and Presentation Matters" in this release for more information. |
(2) | Represents adjustments for impact of the biosimilars divestitures in November 2022 on an operational basis. See "Certain Key Terms and Presentation Matters" in this release for more information. |
(3) | Non-GAAP financial measures. See "Non-GAAP Financial Measures" for additional information. |
(4) | Excluding the impact of transaction costs primarily related to the divestitures and the eye care acquisitions of |
Nine Months Ended | |||||||||
September 30, | |||||||||
(Unaudited; in millions, except %s) | 2023 | 2022 | Reported | Operational | Divestiture- | ||||
Total Net Sales | $ 11,562.5 | $ 12,351.0 | (6) % | (4) % | — % | ||||
Developed Markets | 6,932.7 | 7,386.7 | (6) % | (6) % | — % | ||||
Emerging Markets | 1,932.5 | 2,035.0 | (5) % | 2 % | 4 % | ||||
JANZ | 1,052.2 | 1,233.9 | (15) % | (8) % | (8) % | ||||
1,645.1 | 1,695.4 | (3) % | 2 % | 2 % | |||||
Net Sales by Product Category | |||||||||
Brands | (2) % | — % | — % | ||||||
Complex Gx | 449.7 | 1,065.8 | (58) % | (58) % | (19) % | ||||
Generics | 3,714.7 | 3,707.7 | — % | 3 % | 4 % | ||||
(7) % | |||||||||
41.8 % | 42.2 % | ||||||||
Adjusted Gross Profit (3) | (6) % | ||||||||
Adjusted Gross Margin (3) | 59.7 % | 59.5 % | |||||||
$ 820.3 | (23) % | ||||||||
Adjusted Net Earnings (3) | (14) % | ||||||||
EBITDA (3) | (9) % | ||||||||
Adjusted EBITDA (3) | (12) % | (10) % | (7) % | ||||||
(17) % | |||||||||
Capital expenditures | 211.5 | 252.3 | (16) % | ||||||
Free cash flow (3) (4) | (18) % | ||||||||
___________ | |
(1) | Represents operational change for net sales and adjusted EBITDA which excludes the impacts of foreign currency translation. See "Certain Key Terms and Presentation Matters" in this release for more information. |
(2) | Represents adjustments for impact of the biosimilars divestitures in November 2022 on an operational basis and a reclassification. See "Certain Key Terms and Presentation Matters" in this release for more information. |
(3) | Non-GAAP financial measures. See "Non-GAAP Financial Measures" for additional information. |
(4) | Excluding the impact of transaction costs primarily related to the divestitures and the eye care acquisitions of |
Financial Highlights
- Third quarter 2023 total net sales totaled
$3.93 billion 1% on a divestiture-adjusted operational basis (as defined in "Certain Key Terms and Presentation Matters" below) compared to Q3 2022 results. - Brands performed in line with expectations, reflecting solid year-over-year performance in key brands including Yupelri® and Dymista® and sales from Tyrvaya®.
- Complex generics performed slightly below expectations due to phasing of new product launches.
- Generics, which include diversified product forms such as oral solids, injectables, transdermals and topicals, performed ahead of expectations due to solid performance across broader portfolio in Developed and Emerging Markets.
- The Company generated approximately
$135 million $345 million U.S. We expect to deliver more than$450 million - The Company had
U.S. GAAP net cash provided by operating activities of$834 million $2.32 billion $738 million $2.11 billion U.S. GAAP net cash provided by operating activities and free cash flow for the third quarter included approximately$48 million $79 million - The Company paid down
$23 million $750 million
Certain Key Terms and Presentation Matters
New product sales, new product launches or new product revenues: Refers to revenue from new products launched in 2023 and the carryover impact of new products, including business development, launched within the last twelve months.
Operational change: Refers to constant currency percentage changes and is derived by translating amounts for the current period at prior year comparative period exchange rates, and in doing so shows the percentage change from 2023 constant currency net sales, revenues and adjusted EBITDA to the corresponding amount in the prior year.
Divestiture-adjusted operational change: Refers to operational changes, further adjusted for the impact of the biosimilars divestiture in November 2022 by excluding biosimilars net sales from 2022 periods, and a reclassification to conform prior year-to-date amounts to current year presentation of divestiture-adjusted operational net sales.
SG&A and R&D TSA reimbursement: Expenses related to TSA services provided to Biocon Biologics are recorded in their respective functional line item; however, reimbursement of those expenses plus the mark-up is included in other (income) expense, net. For comparability purposes, amounts related to the cost reimbursement are reclassified to adjusted SG&A and adjusted R&D. This reclassification has no impact on adjusted net earnings or adjusted EBITDA.
Non-GAAP Financial Measures
This press release includes the presentation and discussion of certain financial information that differs from what is reported under accounting principles generally accepted in
About Viatris
Viatris Inc. (NASDAQ: VTRS) is a global healthcare company uniquely positioned to bridge the traditional divide between generics and brands, combining the best of both to more holistically address healthcare needs globally. With a mission to empower people worldwide to live healthier at every stage of life, we provide access at scale. In 2022 alone, we supplied high-quality medicines to approximately 1 billion patients around the world. With our exceptionally extensive and diverse portfolio of medicines, a one-of-a-kind global supply chain designed to reach more people when and where they need them, and the scientific expertise to address some of the world's most enduring health challenges, access takes on deep meaning at Viatris. We have the ability to touch all of life's moments, from birth to end of life, acute conditions to chronic diseases. We are headquartered in the
Forward-Looking Statements
This release contains "forward-looking statements". These statements are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Such forward-looking statements may include, without limitation, 2023 financial guidance; Viatris reaffirms full-year 2023 adjusted EBITDA and free cash flow guidance ranges; strong results signal continuation of growth journey with second consecutive quarter of year-over-year operational revenue growth on a divestiture-adjusted basis; revises full-year total revenues guidance range solely to reflect the expected negative impact of foreign exchange; on track to complete all planned divestitures by the end of the first half of 2024, subject to regulatory approvals, completion of any consultations with employee representatives (where applicable), receipt of required consents and other closing conditions, including, in the case of the API business divestiture, a financing condition; these results are an indication of the continuing momentum we are building as we prepare to bring Phase 1 of our strategic plan to successful completion; operationally, we are continuing to see strong performance globally across our businesses; we are now shifting our focus to Phase 2 and to adding to the strength of our stable base by building the business in areas with the greatest potential for growth, patient impact and shareholder value; as a result of our continued advancement of our robust and deep pipeline, we are on track to deliver
ViatrisInc. and Subsidiaries Condensed Consolidated Statements of Operations (Unaudited) | |||||||
Three Months Ended | Nine Months Ended | ||||||
September 30, | September 30, | ||||||
(In millions, except per share amounts) | 2023 | 2022 | 2023 | 2022 | |||
Revenues: | |||||||
Net sales | $ 3,933.9 | $ 4,067.4 | $ 11,562.5 | $ 12,351.0 | |||
Other revenues | 8.0 | 10.8 | 27.1 | 35.7 | |||
Total revenues | 3,941.9 | 4,078.2 | 11,589.6 | 12,386.7 | |||
Cost of sales | 2,250.6 | 2,329.8 | 6,747.5 | 7,163.8 | |||
Gross profit | 1,691.3 | 1,748.4 | 4,842.1 | 5,222.9 | |||
Operating expenses: | |||||||
Research and development | 211.2 | 174.9 | 602.4 | 479.8 | |||
Acquired IPR&D | 1.0 | — | 11.2 | — | |||
Selling, general and administrative | 1,053.5 | 1,017.3 | 3,044.3 | 2,913.7 | |||
Litigation settlements and other contingencies, net | (26.1) | (3.9) | (36.5) | 13.2 | |||
Total operating expenses | 1,239.6 | 1,188.3 | 3,621.4 | 3,406.7 | |||
Earnings from operations | 451.7 | 560.1 | 1,220.7 | 1,816.2 | |||
Interest expense | 141.5 | 153.2 | 432.2 | 445.3 | |||
Other (income) expense, net | (92.0) | (20.6) | (269.4) | 26.6 | |||
Earnings before income taxes | 402.2 | 427.5 | 1,057.9 | 1,344.3 | |||
Income tax provision | 70.6 | 73.2 | 237.6 | 276.9 | |||
Net earnings | $ 331.6 | $ 354.3 | $ 820.3 | $ 1,067.4 | |||
Earnings per share attributable to Viatris Inc. shareholders | |||||||
Basic | $ 0.28 | $ 0.29 | $ 0.68 | $ 0.88 | |||
Diluted | $ 0.27 | $ 0.29 | $ 0.68 | $ 0.88 | |||
Weighted average shares outstanding: | |||||||
Basic | 1,199.5 | 1,212.5 | 1,200.4 | 1,211.8 | |||
Diluted | 1,207.6 | 1,218.1 | 1,205.6 | 1,216.1 | |||
ViatrisInc. and Subsidiaries Condensed Consolidated Balance Sheets (Unaudited) | |||
(In millions) | September 30, | December 31, | |
ASSETS | |||
Assets | |||
Current assets: | |||
Cash and cash equivalents | $ 1,309.6 | $ 1,259.9 | |
Accounts receivable, net | 3,738.5 | 3,814.5 | |
Inventories | 3,671.9 | 3,519.5 | |
Prepaid expenses and other current assets | 1,784.7 | 1,811.2 | |
Assets held for sale | 427.3 | 230.3 | |
Total current assets | 10,932.0 | 10,635.4 | |
Intangible assets, net | 21,280.5 | 22,607.1 | |
Goodwill | 10,278.1 | 10,425.8 | |
Other non-current assets | 6,252.0 | 6,353.9 | |
Total assets | $ 48,742.6 | $ 50,022.2 | |
LIABILITIES AND EQUITY | |||
Liabilities | |||
Current portion of long-term debt and other long-term obligations | $ 1,307.4 | $ 1,259.1 | |
Liabilities held for sale | 14.3 | — | |
Other current liabilities | 5,512.3 | 5,487.1 | |
Long-term debt | 17,076.9 | 18,015.2 | |
Other non-current liabilities | 3,966.1 | 4,188.5 | |
Total liabilities | 27,877.0 | 28,949.9 | |
Shareholders' equity | 20,865.6 | 21,072.3 | |
Total liabilities and equity | $ 48,742.6 | $ 50,022.2 | |
Viatris Inc. and Subsidiaries | |||||
Key Product Net Sales, on a Consolidated Basis | |||||
(Unaudited) | |||||
Three months ended | Nine months ended | ||||
(In millions) | 2023 | 2022 | 2023 | 2022 | |
Select Key Global Products | |||||
Lipitor ® | $ 381.6 | $ 420.4 | $ 1,179.5 | ||
Norvasc ® | 175.5 | 189.3 | 560.6 | 600.1 | |
Lyrica ® | 141.7 | 156.5 | 423.1 | 483.9 | |
EpiPen® Auto-Injectors | 131.9 | 114.4 | 355.2 | 309.7 | |
Viagra ® | 110.5 | 117.0 | 336.5 | 361.9 | |
Celebrex ® | 84.7 | 82.2 | 255.5 | 253.4 | |
Creon ® | 77.5 | 76.4 | 224.3 | 226.5 | |
Effexor ® | 65.5 | 64.2 | 194.9 | 215.4 | |
Zoloft ® | 62.7 | 53.1 | 173.7 | 188.7 | |
Xalabrands | 47.9 | 51.0 | 145.0 | 146.7 | |
Select Key Segment Products | |||||
Influvac ® | $ 137.2 | $ 159.3 | $ 137.5 | $ 178.3 | |
Yupelri ® | 58.3 | 53.4 | 160.3 | 146.1 | |
Dymista ® | 44.1 | 38.6 | 155.0 | 138.0 | |
Amitiza ® | 37.7 | 39.4 | 115.8 | 125.3 | |
Xanax ® | 28.2 | 38.3 | 119.7 | 115.5 | |
____________ | |
(a) | The Company does not disclose net sales for any products considered competitively sensitive. |
(b) | Products disclosed may change in future periods, including as a result of seasonality, competition or new product launches. |
(c) | Amounts for the three and nine months ended September 30, 2023 include the impact of foreign currency translations compared to the prior year period. |
ViatrisInc. and Subsidiaries Reconciliation of Non-GAAP Financial Measures (Unaudited) | |||||||
Reconciliation of | |||||||
Below is a reconciliation of | |||||||
Three Months Ended | Nine Months Ended | ||||||
(In millions) | 2023 | 2022 | 2023 | 2022 | |||
$ 331.6 | $ 354.3 | $ 820.3 | $ 1,067.4 | ||||
Purchase accounting related amortization (primarily included in cost of sales) (a) | 602.0 | 626.7 | 1,864.6 | 1,930.5 | |||
Litigation settlements and other contingencies, net | (26.1) | (3.9) | (36.5) | 13.2 | |||
Interest expense (primarily amortization of premiums and discounts on long term debt) | (10.7) | (10.0) | (31.5) | (36.8) | |||
Acquisition and divestiture-related costs (primarily included in SG&A) (b) | 115.7 | 99.2 | 230.1 | 306.3 | |||
Restructuring related costs (c) | 14.9 | 15.0 | 98.7 | 42.0 | |||
Share-based compensation expense | 43.1 | 29.1 | 124.9 | 86.8 | |||
Other special items included in: | |||||||
Cost of sales (d) | 16.7 | 68.9 | 91.9 | 150.4 | |||
Research and development expense | 0.3 | — | 2.7 | 0.9 | |||
Selling, general and administrative expense | 2.7 | 19.9 | 34.0 | 44.3 | |||
Other income, net (e) | (26.4) | (6.3) | (114.0) | (8.2) | |||
Tax effect of the above items and other income tax related items (f) | (111.0) | (129.4) | (294.1) | (342.7) | |||
Adjusted net earnings | $ 952.8 | $ 1,063.5 | $ 2,791.1 | $ 3,254.1 | |||
____________ | |
Significant items include the following: | |
(a) | For the nine months ended September 30, 2023, charges include an intangible asset charge of approximately |
(b) | Acquisition and divestiture-related costs consist primarily of transaction costs including legal and consulting fees and integration activities. |
(c) | For the three and nine months ended September 30, 2023, charges include approximately |
(d) | For the three and nine months ended September 30, 2023, charges include incremental manufacturing variances at plants in the 2020 restructuring program of approximately |
(e) | For the three months ended September 30, 2023, includes a gain of approximately |
(f) | Adjusted for changes for uncertain tax positions. |
Reconciliation of | |||||||
Below is a reconciliation of | |||||||
Three Months Ended | Nine Months Ended | ||||||
September 30, | September 30, | ||||||
(In millions) | 2023 | 2022 | 2023 | 2022 | |||
$ 331.6 | $ 354.3 | $ 820.3 | $ 1,067.4 | ||||
Add adjustments: | |||||||
Income tax provision | 70.6 | 73.2 | 237.6 | 276.9 | |||
Interest expense (a) | 141.5 | 153.2 | 432.2 | 445.3 | |||
Depreciation and amortization (b) | 679.4 | 699.5 | 2,096.1 | 2,157.8 | |||
EBITDA | $ 1,223.1 | $ 1,280.2 | $ 3,586.2 | $ 3,947.4 | |||
Add / (deduct) adjustments: | |||||||
Share-based compensation expense | 43.1 | 29.1 | 124.9 | 86.8 | |||
Litigation settlements and other contingencies, net | (26.1) | (3.9) | (36.5) | 13.2 | |||
Restructuring, acquisition and divestiture-related and other special items (c) | 120.0 | 192.4 | 332.1 | 518.8 | |||
Adjusted EBITDA | $ 1,360.1 | $ 1,497.8 | $ 4,006.7 | $ 4,566.2 | |||
___________ | |
(a) | Includes amortization of premiums and discounts on long-term debt. |
(b) | Includes purchase accounting related amortization. |
(c) | See items detailed in the Reconciliation of |
Summary of Total Revenues by Segment | |||||||||||||||||||
Three Months Ended | |||||||||||||||||||
September 30, | |||||||||||||||||||
(In millions, except %s) | 2023 | 2022 | % | 2023 | 2023 | Constant | 2022 | 2022 | Divestiture- | ||||||||||
Net sales | |||||||||||||||||||
Developed Markets | (1) % | $ (85.0) | $ 2,323.5 | (4) % | $ 162.9 | $ 2,268.6 | 2 % | ||||||||||||
548.4 | 574.0 | (4) % | 23.7 | 572.1 | — % | 0.2 | 573.8 | — % | |||||||||||
JANZ | 334.5 | 383.0 | (13) % | 18.9 | 353.4 | (8) % | 5.1 | 377.9 | (6) % | ||||||||||
Emerging Markets | 642.5 | 678.9 | (5) % | 35.8 | 678.3 | — % | 12.8 | 666.1 | 2 % | ||||||||||
Total net sales | (3) % | $ (6.6) | $ 3,927.3 | (3) % | $ 181.0 | $ 3,886.4 | 1 % | ||||||||||||
Other revenues (7) | 8.0 | 10.8 | NM | (0.3) | 7.7 | NM | |||||||||||||
Consolidated total revenues (8) | (3) % | $ (6.9) | $ 3,935.0 | (3) % | |||||||||||||||
Nine Months Ended | |||||||||||||||||||
September 30, | |||||||||||||||||||
(In millions, except %s) | 2023 | 2022 | % Change | 2023 | 2023 | Constant | 2022 | Other (4) | 2022 | Divestiture- | |||||||||
Net sales | |||||||||||||||||||
Developed Markets | (6) % | $ (23.7) | $ 6,909.0 | (6) % | $ 449.4 | $ 13.9 | $ 6,923.4 | — % | |||||||||||
1,645.1 | 1,695.4 | (3) % | 85.1 | 1,730.2 | 2 % | 0.6 | (4.2) | 1,699.0 | 2 % | ||||||||||
JANZ | 1,052.2 | 1,233.9 | (15) % | 77.6 | 1,129.8 | (8) % | 14.7 | (9.7) | 1,228.9 | (8) % | |||||||||
Emerging Markets | 1,932.5 | 2,035.0 | (5) % | 143.1 | 2,075.6 | 2 % | 42.8 | — | 1,992.2 | 4 % | |||||||||
Total net sales | $ 11,562.5 | $ 12,351.0 | (6) % | $ 282.1 | $ 11,844.6 | (4) % | $ 507.5 | $ — | $ 11,843.5 | — % | |||||||||
Other revenues (7) | 27.1 | 35.7 | NM | 0.1 | 27.2 | NM | |||||||||||||
Consolidated total revenues (8) | $ 11,589.6 | $ 12,386.7 | (6) % | $ 282.2 | $ 11,871.8 | (4) % | |||||||||||||
____________ | |
(1) | Currency impact is shown as unfavorable (favorable). |
(2) | The constant currency percentage change is derived by translating net sales or revenues for the current period at prior year comparative period exchange rates, and in doing so shows the percentage change from 2023 constant currency net sales or revenues to the corresponding amount in the prior year. |
(3) | Represents biosimilars net sales in the relevant period. |
(4) | Represents a reclassification to conform prior year amounts to current year presentation of divestiture-adjusted operational net sales. |
(5) | Represents |
(6) | See "Certain Key Terms and Presentation Matters" in this release for more information. |
(7) | For the three months ended September 30, 2023, other revenues in Developed Markets, JANZ, and Emerging Markets were approximately |
(8) | Amounts exclude intersegment revenue which eliminates on a consolidated basis. |
Reconciliation of Income Statement Line Items | |||||||
(Unaudited) | |||||||
Three Months Ended | Nine Months Ended | ||||||
September 30, | September 30, | ||||||
(In millions, except %s) | 2023 | 2022 | 2023 | 2022 | |||
$ 2,250.6 | $ 2,329.8 | $ 6,747.5 | $ 7,163.8 | ||||
Deduct: | |||||||
Purchase accounting related amortization | (602.0) | (626.7) | (1,864.7) | (1,930.4) | |||
Acquisition and divestiture-related items | (14.1) | (16.3) | (26.7) | (41.1) | |||
Restructuring related costs | (9.1) | (8.6) | (88.9) | (28.4) | |||
Share-based compensation expense | (0.7) | (0.4) | (2.2) | (1.2) | |||
Other special items | (16.7) | (68.9) | (91.9) | (150.4) | |||
Adjusted cost of sales | $ 1,608.0 | $ 1,608.9 | $ 4,673.1 | $ 5,012.3 | |||
Adjusted gross profit (a) | $ 2,333.9 | $ 2,469.3 | $ 6,916.5 | $ 7,374.4 | |||
Adjusted gross margin (a) | 59 % | 61 % | 60 % | 60 % | |||
Three Months Ended | Nine Months Ended | ||||||
September 30, | September 30, | ||||||
(In millions, except %s) | 2023 | 2022 | 2023 | 2022 | |||
$ 211.2 | $ 174.9 | $ 602.4 | $ 479.8 | ||||
Deduct: | |||||||
Acquisition and divestiture-related costs | (2.2) | (2.6) | (9.2) | (6.3) | |||
Share-based compensation expense | (1.5) | (1.1) | (4.0) | (4.1) | |||
SG&A and R&D TSA reimbursement (c) | (8.6) | — | (27.0) | — | |||
Other special items | (0.3) | — | (2.7) | (0.9) | |||
Adjusted R&D | $ 198.6 | $ 171.2 | $ 559.5 | $ 468.5 | |||
Adjusted R&D as % of total revenues | 5 % | 4 % | 5 % | 4 % | |||
Three Months Ended | Nine Months Ended | ||||||
September 30, | September 30, | ||||||
(In millions, except %s) | 2023 | 2022 | 2023 | 2022 | |||
$ 1,053.5 | $ 1,017.3 | $ 3,044.3 | $ 2,913.7 | ||||
Deduct: | |||||||
Acquisition and divestiture-related costs | (99.4) | (80.4) | (194.1) | (258.9) | |||
Restructuring and related costs | (5.8) | (6.4) | (9.8) | (13.6) | |||
Purchase accounting amortization and other related items | — | — | — | (0.1) | |||
Share-based compensation expense | (40.9) | (27.5) | (118.7) | (81.5) | |||
SG&A and R&D TSA reimbursement (c) | (27.6) | — | (79.8) | — | |||
Other special items and reclassifications | (2.7) | (19.9) | (34.0) | (44.3) | |||
Adjusted SG&A | $ 877.1 | $ 883.1 | $ 2,607.9 | $ 2,515.3 | |||
Adjusted SG&A as % of total revenues | 22 % | 22 % | 23 % | 20 % | |||
Three Months Ended | Nine Months Ended | ||||||
September 30, | September 30, | ||||||
(In millions) | 2023 | 2022 | 2023 | 2022 | |||
$ 1,239.6 | $ 1,188.3 | $ 3,621.4 | $ 3,406.7 | ||||
Add / (Deduct): | |||||||
Litigation settlements and other contingencies, net | 26.1 | 3.9 | 36.5 | (13.2) | |||
R&D adjustments | (12.6) | (3.7) | (42.9) | (11.3) | |||
SG&A adjustments | (176.4) | (134.2) | (436.4) | (398.4) | |||
Adjusted total operating expenses | $ 1,076.7 | $ 1,054.3 | $ 3,178.6 | $ 2,983.8 | |||
Adjusted earnings from operations (b) | $ 1,257.2 | $ 1,415.0 | $ 3,737.9 | $ 4,390.6 | |||
Three Months Ended | Nine Months Ended | ||||||
September 30, | September 30, | ||||||
(In millions) | 2023 | 2022 | 2023 | 2022 | |||
$ 141.5 | $ 153.2 | $ 432.2 | $ 445.3 | ||||
Add / (Deduct): | |||||||
Accretion of contingent consideration liability | (2.0) | (1.8) | (6.3) | (5.6) | |||
Amortization of premiums and discounts on long-term debt | 13.7 | 12.8 | 40.8 | 45.7 | |||
Other special items | (1.0) | (1.1) | (3.0) | (3.3) | |||
Adjusted interest expense | $ 152.2 | $ 163.1 | $ 463.7 | $ 482.1 | |||
Three Months Ended | Nine Months Ended | ||||||
September 30, | September 30, | ||||||
(In millions) | 2023 | 2022 | 2023 | 2022 | |||
$ (92.0) | $ (20.6) | $ (269.4) | $ 26.6 | ||||
Add / (Deduct): | |||||||
Fair value adjustments on non-marketable equity investments (d) | 19.1 | — | 115.1 | — | |||
SG&A and R&D TSA reimbursement (c) | 36.2 | — | 106.8 | — | |||
Other items | 7.3 | 6.3 | (1.1) | 8.2 | |||
Adjusted other (income) expense, net | $ (29.4) | $ (14.3) | $ (48.6) | $ 34.8 | |||
Three Months Ended | Nine Months Ended | ||||||
September 30, | September 30, | ||||||
(In millions, except %s) | 2023 | 2022 | 2023 | 2022 | |||
$ 402.2 | $ 427.5 | $ 1,057.9 | $ 1,344.3 | ||||
Total pre-tax non-GAAP adjustments | 732.1 | 838.5 | 2,264.8 | 2,529.3 | |||
Adjusted earnings before income taxes | $ 1,134.3 | $ 1,266.0 | $ 3,322.7 | $ 3,873.6 | |||
$ 70.6 | $ 73.2 | $ 237.6 | $ 276.9 | ||||
Adjusted tax expense | 110.9 | 129.4 | 294.0 | 342.7 | |||
Adjusted income tax provision | $ 181.5 | $ 202.6 | $ 531.6 | $ 619.6 | |||
Adjusted effective tax rate | 16.0 % | 16.0 % | 16.0 % | 16.0 % | |||
___________ | |
(a) | |
(b) | |
(c) | Refer to "Certain Key Terms and Presentation Matters" section in this release for more information on reclassifications related to TSA reimbursements. |
(d) | For the three months ended September 30, 2023, includes a gain of approximately |
Reconciliation of Estimated 2023 U.S. GAAP Net Cash Provided by Operating Activities to Free Cash Flow
(Unaudited) | |
A reconciliation of the estimated 2023 U.S. GAAP Net Cash provided by Operating Activities to Free Cash Flow is | |
(In millions) | |
Estimated | |
Less: Capital Expenditures | |
Free Cash Flow (a) | |
___________ | |
(a) | Includes the full year expected performance for the planned divestitures and excludes any potential related costs, such as taxes and transaction costs, as well as any similar costs related to the eye care acquisitions. Also excludes any acquired IPR&D for unsigned deals. |
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/viatris-reports-strong-financial-and-operational-results-for-the-third-quarter-2023-and-reaffirms-full-year-2023-adjusted-ebitda-and-free-cash-flow-guidance-ranges1-301980611.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/viatris-reports-strong-financial-and-operational-results-for-the-third-quarter-2023-and-reaffirms-full-year-2023-adjusted-ebitda-and-free-cash-flow-guidance-ranges1-301980611.html
SOURCE Viatris Inc.