Volition's Nu.Q® NETs Assay Demonstrates Promising Results in COVID-19 Risk Stratification and Disease Monitoring
VolitionRx Limited (NYSE AMERICAN: VNRX) announced promising results from its Nu.Q® NETs assay in COVID-19 studies presented at the ISTH Congress 2021. This assay correlates with disease severity, predicting future COVID-19 outcomes and assisting in risk stratification for treatment. The findings suggest potential in monitoring disease progression, particularly in intensive care scenarios. Ongoing large studies on COVID-19, sepsis, and more are expected to yield further data shortly, indicating a strong pipeline for Volition's diagnostic capabilities.
- Promising results from Nu.Q® NETs assay correlate with COVID-19 severity.
- Assay predicts future disease outcomes and aids in treatment risk stratification.
- Ongoing large studies indicate strong future data pipeline.
- None.
Insights
Analyzing...
AUSTIN, Texas, July 22, 2021 /PRNewswire/ -- VolitionRx Limited (NYSE AMERICAN: VNRX) ("Volition"), a multi-national epigenetics company developing simple, easy to use, cost effective blood tests to help diagnose a range of cancers and other diseases in both humans and animals, has been working with researchers at two leading NHS Foundation Trusts on two studies in COVID-19 for which abstracts were released this week at the International Society on Thrombosis and Haemostasis (ISTH) Congress 2021.
Dr. Jake Micallef, Chief Scientific Officer of Volition said "We believe our Nu.Q® NETs assay will have wide applicability for monitoring diseases with a NETs component (such as COVID-19, sepsis, autoimmune diseases and cancer) and potentially to risk stratify patients for treatment selection. We have further large studies in progress in COVID-19, sepsis and other diseases as well as studies in potential use as a companion diagnostic in sepsis. I look forward to the completion of ongoing studies and the publication of further data in the coming months."
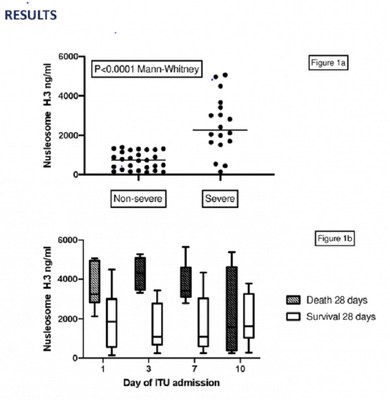
Volition previously reported preliminary results demonstrating that its Nu.Q® NETs assay correlated well with (current) COVID-19 disease severity. The Company now reports early-stage data with the same assay showing that results on admission could predict future COVID-19 disease severity and that serial results correlate with disease progression.
Commenting on the poster presentation entitled "Circulating Nucleosomes Immunoassay: Evaluating a Clinically Applicable Test to Risk Stratify COVID-19 and Target Anticoagulation, including the results show above," lead author Dr. Catherine Rea, Consultant Haematologist and Researcher, said "The Nu.Q® NETs biomarker results taken on hospital admission in this study correlated with COVID-19 disease severity and were predictive of whether patients required care in a general ward or organ support in an intensive care ward. The study results also indicated that elevated values of Nu.Q® H3.1 could predict poor outcomes in patients admitted to Intensive Care, including an association with 28-day mortality, and may be of value in risk stratifying patients for treatments such as therapeutic anticoagulation, as well as in monitoring patient response to treatment."
Commenting on the poster presentation entitled "Identifying Tools to Track Hypercoagulability in COVID-19 Patients. Exploring Global Haemostasis (ROTEM) and Neutrophil Extracellular Traps (NETs) Immunoassays," lead author Dr. Sophia Stanford, Lead Scientist, said, "Whilst this is a small exploratory study, the Nu.Q® NETs H3.1 biomarker values closely tracked the clinical course of COVID-19 patients admitted directly to Intensive Care, admitted to a general ward or admitted to a general ward and then transferred to Intensive Care during their hospital stay. These findings suggest the Nu.Q® H3.1 assay may be able to risk stratify COVID-19 patients on admission and monitor disease progression in individual patients."
To view or download the poster given by Dr. Rea click here.
To view or download the poster given by Dr. Stanford click here.
For further details please contact mediarelations@volition.com.
About Volition
Volition is a multi-national epigenetics company developing simple, easy to use, cost effective blood tests to help diagnose a range of cancers and other diseases. Early diagnosis has the potential to not only prolong the life of patients, but also to improve their quality of life. The tests are based on the science of Nucleosomics™, which is the practice of identifying and measuring nucleosomes in the bloodstream or other bodily fluid - an indication that disease is present. Volition is primarily focused on human diagnostics but also has a subsidiary focused on animal diagnostics.
Volition's research and development activities are centered in Belgium, with a small laboratory in California and additional offices in Texas, London and Singapore, as the company focuses on bringing its diagnostic products to market.
For more information about Volition, visit Volition's website volition.com or connect with us via:
Twitter: https://twitter.com/volitionrx
LinkedIn: https://www.linkedin.com/company/volitionrx
Facebook: https://www.facebook.com/VolitionRx/
YouTube: https://www.youtube.com/user/VolitionRx
The contents found at Volition's website address, Twitter, LinkedIn, Facebook, and YouTube are not incorporated by reference into this document and should not be considered part of this document. The addresses for Volition's website, Twitter, LinkedIn, Facebook, and YouTube are included in this document as inactive textual references only.
Media / Investor Contacts
Louise Batchelor, Volition +44 (0)7557 774620 | Scott Powell, Volition investorrelations@volition.com +1 (646) 650 1351 |
Jen Lewis, Mind&Matter +44 (0)7809 867943 | Joseph Green, Edison Advisors +1 (646) 653 7030 |
Safe Harbor Statement
Statements in this press release may be "forward-looking statements" within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, that concern matters that involve risks and uncertainties that could cause actual results to differ materially from those anticipated or projected in the forward-looking statements. Words such as "expects," "anticipates," "intends," "plans," "aims," "targets," "believes," "seeks," "estimates," "optimizing," "potential," "goal," "suggests," "could," "would," "should," "may," "will" and similar expressions identify forward-looking statements. These forward-looking statements relate to the timing, completion and delivery of data from clinical studies, the effectiveness of Volition's blood-based diagnostic and prognostic tests, Volition's ability to develop and successfully commercialize such test platforms for early detection of cancer and other diseases as well as serving as a diagnostic or prognostic tool for COVID-19, and the timing of product launches and publications. Volition's actual results may differ materially from those indicated in these forward-looking statements due to numerous risks and uncertainties, including, without limitation, results of studies testing the efficacy of its tests. For instance, if Volition fails to develop and commercialize diagnostic or prognostic products, it may be unable to execute its plan of operations. Other risks and uncertainties include Volition's failure to obtain necessary regulatory clearances or approvals to distribute and market future products; a failure by the marketplace to accept the products in Volition's development pipeline or any other diagnostic or prognostic products Volition might develop; Volition's failure to secure adequate intellectual property protection; Volition will face fierce competition and Volition's intended products may become obsolete due to the highly competitive nature of the diagnostics market and its rapid technological change; downturns in domestic and foreign economies; and other risks identified in Volition's most recent Annual Report on Form 10-K and Quarterly Reports on Form 10-Q, as well as other documents that Volition files with the Securities and Exchange Commission. These statements are based on current expectations, estimates and projections about Volition's business based, in part, on assumptions made by management. These statements are not guarantees of future performance and involve risks, uncertainties and assumptions that are difficult to predict. Forward-looking statements are made as of the date of this release, and, except as required by law, Volition does not undertake an obligation to update its forward-looking statements to reflect future events or circumstances.
Nucleosomics™ and Nu.Q® and their respective logos are trademarks and/or service marks of VolitionRx Limited and its subsidiaries. All other trademarks, service marks and trade names referred to in this press release are the property of their respective owners.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/volitions-nuq-nets-assay-demonstrates-promising-results-in-covid-19-risk-stratification-and-disease-monitoring-301339342.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/volitions-nuq-nets-assay-demonstrates-promising-results-in-covid-19-risk-stratification-and-disease-monitoring-301339342.html
SOURCE VolitionRx Limited