United Therapeutics Announces World’s First Successful Xenothymokidney Transplant
The first living recipient of a UThymoKidney, in conjunction with a heart pump implant, is recovering after a successful transplant
This transplant builds on two successful UHeart transplants completed in 2022 and 2023
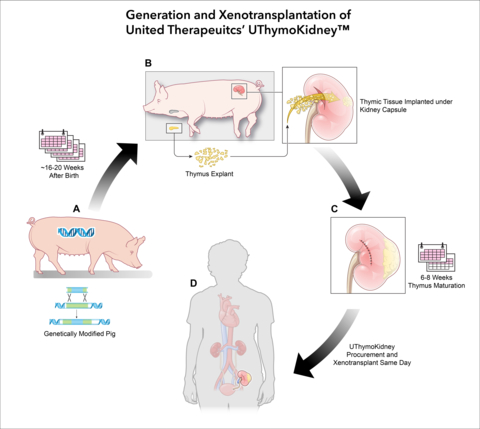
A) Genetically modified source pig, 16 to 20 weeks old. B) The thymus is then explanted from the source genetically modified pig, and the thymic tissue is implanted under its kidney capsule. C) The thymus tissue matures under the kidney capsule for six to eight weeks. D) The UThymoKidney is procured and transplanted into human recipient. (Photo: Business Wire)
- The first-ever transplant of a xenothymokidney into a living human recipient;
- The first-ever combined mechanical heart pump and organ transplant; and
- The first-ever xenotransplant into a living human using only FDA-approved immunosuppressive medicines
The transplant is the third xenotransplant using United Therapeutics’ xeno organs, following two successful UHeart™ transplants at the University of Maryland Medicine in 2022 and 2023.
The transplant was authorized by
United Therapeutics’ xenothymokidney, known by the proposed trade name UThymoKidney, is an investigational-stage xenokidney from a pig with a single genetic edit, together with tissue from the same pig’s thymus. The use of the pig’s thymus tissue is intended to condition the recipient human’s immune system to recognize the UThymoKidney as “self” and reduce the likelihood of rejection.
The single genetic modification in the pig is the inactivation, or “knock-out”, of the gene responsible for the synthesis of alpha-gal, a sugar on the surface of cells that can cause the immediate rejection of an organ when transplanted into the human body. Because tissues from pigs containing this modification do not contain detectable levels of alpha-gal, United Therapeutics refers to materials derived from this pig as GalSafe®.
The GalSafe pig was developed by Revivicor, Inc., a subsidiary of United Therapeutics. In December 2020, this pig line was approved by the FDA for use as human food or as a potential source for biomedical purposes, with this being the first investigational biomedical use in a living human.
“This historic transplant builds on the base of knowledge that the teams at United Therapeutics and our academic collaborators have established over the past two decades and demonstrates the potential utility for xeno organs to revolutionize the way patients with end-stage organ disease are managed in the future,” said Leigh Peterson, Ph.D., Executive Vice President, Product Development & Xenotransplantation at United Therapeutics. “We look forward to continuing our dialogue with the FDA with the goal of starting human clinical studies for xenotransplantation in 2025.”
According to the
“I am pleased and impressed that decades of research into expanding the supply of kidneys have resulted in this historic, successful xenokidney transplant using United Therapeutics' gene editing and thymokidney technology,” said Dr. Louis Sullivan, Secretary of the United States Department of Health and Human Services in President George H.W. Bush’s administration, member of the United Therapeutics Board of Directors, and Chair of its Scientific Advisory Board.
“I am so proud of the many scientists and surgeons working with United Therapeutics on its xenotransplantation programs,” said Gov. Tommy Thompson, Secretary of the United States Department of Health and Human Services in President George W. Bush's administration and member of United Therapeutics' Board of Directors. “This major breakthrough is a revolutionary step forward in our quest to create an unlimited supply of transplantable organs.”
United Therapeutics’ organ manufacturing efforts consist of four platforms – xenotransplantation, regenerative medicine, 3D organ bioprinting, and bio-artificial organs - encompassing four different organs – hearts, kidneys, livers, and lungs. These groundbreaking programs are intended to address the ongoing shortage of transplantable organs for patients with end stage organ disease.
United Therapeutics initiated its xenotransplantation research work in 2011 and currently employs close to 50 scientists and support staff advancing xenotransplant science with three different organ programs: the UHeart xenoheart, the UThymoKidney, a one-gene modified kidney and thymus, and the UKidney™, a 10-gene modified kidney. In 2024, the company inaugurated the world’s first clinical-scale designated pathogen-free facility in
To date, 11 xenotransplantation procedures using United Therapeutics' UHearts, UThymoKidneys, and UKidneys have been performed in living and brain-dead human recipients: two living human recipients of UHearts, one living recipient of a UThymoKidney, six brain-dead UKidney and UThymoKidney recipients, and two brain-dead UHeart recipients. United Therapeutics has built on its history of innovation in xenotransplantation with strong research collaborations with top academic medical centers including NYU Langone Health, the University of Maryland Medicine, Johns Hopkins Medicine, and the University of
United Therapeutics is preparing for clinical trials of its xenokidney, xenothymokidney, and xenoheart products, following completion of ongoing preclinical studies required by the FDA.
United Therapeutics: Enabling Inspiration
At United Therapeutics, our vision and mission are one. We use our enthusiasm, creativity, and persistence to innovate for the unmet medical needs of our patients and to benefit our other stakeholders. We are bold and unconventional. We have fun; we do good. We are the first publicly traded biotech or pharmaceutical company to take the form of a public benefit corporation. Our public benefit purpose is to provide a brighter future for patients through the development of novel pharmaceutical therapies; and technologies that expand the availability of transplantable organs.
You can learn more about what it means to be a PBC here: unither.com/PBC.
Forward-looking Statements
Statements included in this press release that are not historical in nature are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements include, among others, statements regarding progress toward developing our organ manufacturing programs, including our plans to commence clinical trials of one or more xenotransplantation products in 2025, and our goals of innovating for the unmet medical needs of our patients and to benefit our other stakeholders and furthering our public benefit purpose of developing novel pharmaceutical therapies and technologies that expand the availability of transplantable organs. These forward-looking statements are subject to certain risks and uncertainties, such as those described in our periodic reports filed with the Securities and Exchange Commission, that could cause actual results to differ materially from anticipated results. Consequently, such forward-looking statements are qualified by the cautionary statements, cautionary language and risk factors set forth in our periodic reports and documents filed with the Securities and Exchange Commission, including our most recent Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, and Current Reports on Form 8-K. In particular, our plans to commence clinical studies of one or more xenotransplantation products in 2025 are subject to regulatory clearance, including the completion of preclinical studies to the satisfaction of the FDA, and many other factors that we cannot control. We claim the protection of the safe harbor contained in the Private Securities Litigation Reform Act of 1995 for forward-looking statements. We are providing this information as of April 24, 2024, and assume no obligation to update or revise the information contained in this press release whether as a result of new information, future events, or any other reason.
GALSAFE is a registered trademark of United Therapeutics Corporation and its subsidiaries.
UHEART, UKIDNEY, and UTHYMOKIDNEY are trademarks of United Therapeutics Corporation and its subsidiaries.
View source version on businesswire.com: https://www.businesswire.com/news/home/20240424110823/en/
Dewey Steadman at (202) 919-4097
https://ir.unither.com/contact-uthr/
Source: United Therapeutics Corporation








