Unicycive Therapeutics Announces Initial Positive Patient Satisfaction Findings from Pivotal Clinical Trial of Oxylanthanum Carbonate (OLC)
Unicycive Therapeutics announced positive initial patient satisfaction results from its pivotal clinical trial for oxylanthanum carbonate (OLC) in patients with hyperphosphatemia who have chronic kidney disease on dialysis. The survey, part of the UNI-OLC-201 trial, revealed that 79% of patients preferred OLC over their previous phosphate binders, with 98% finding it easy to take compared to 55% for their prior medication. Additionally, 89% of patients reported satisfaction with OLC versus 49% for their previous therapy. The median daily pill burden was halved when switching to OLC. These findings support the potential best-in-class profile of OLC, and the company plans to file a New Drug Application soon.
- 79% of patients preferred OLC over their prior phosphate binder therapy.
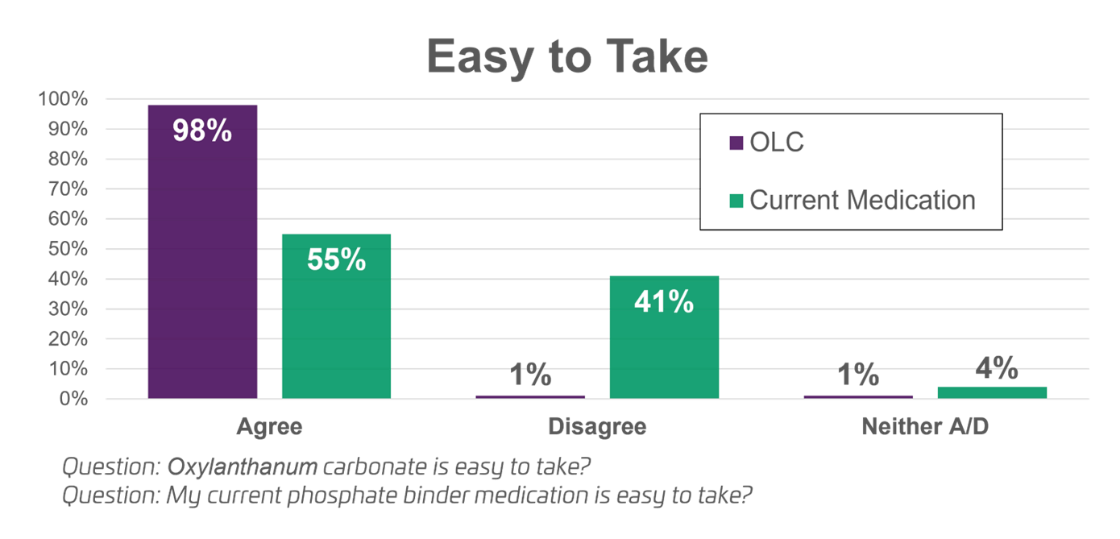
- 98% of patients found OLC easy to take compared to 55% for their prior medication.
- 89% of patients reported satisfaction with OLC versus 49% for their previous therapy.
- Median daily pill burden was reduced by half after switching to OLC.
- The initial findings are preliminary and subject to change based on further detailed analysis.
Insights
The patient satisfaction findings from Unicycive Therapeutics' pivotal trial of oxylanthanum carbonate (OLC) offer promising insights for the treatment of hyperphosphatemia in chronic kidney disease patients. Notably, the trial demonstrated a significant preference for OLC over existing phosphate binders, with
Lowering the median daily pill burden from 6 to 3 pills is a notable improvement. Adherence in chronic diseases like hyperphosphatemia directly impacts clinical outcomes. This reduction in pill burden can lead to higher compliance, thereby improving patient health metrics over the long term.
Retail investors should recognize that these findings validate OLC's market potential, as improving patient satisfaction and adherence are vital for the success of pharmaceutical products. The satisfaction rates and ease of use also suggest a positive reception from both patients and healthcare providers, which can accelerate market penetration upon approval.
From a market perspective, the positive patient satisfaction results for OLC could have substantial implications for Unicycive's positioning within the phosphate binder market. Given that 98% of patients found OLC easy to take, compared to
Investors should consider the potential for rapid uptake if OLC is approved, given the high levels of patient satisfaction and reduced pill burden. Pharmaceutical products that achieve high patient satisfaction are often favored in formulary decisions, leading to better reimbursement scenarios and stronger sales growth.
Unicycive’s strategy to focus on filing a New Drug Application (NDA) and its potential swift market entry also suggests short-term catalysts that could positively affect stock performance. Long-term, the ability to maintain patient adherence and satisfaction could drive sustained revenue growth and market share gains.
– Patients preferred OLC more than 4 to 1 over their prior phosphate binder therapy –
– Median daily pill burden reduced by half after switch to OLC –
LOS ALTOS, Calif., July 10, 2024 (GLOBE NEWSWIRE) -- Unicycive Therapeutics, Inc. (Nasdaq: UNCY), a clinical-stage biotechnology company developing therapies for patients with kidney disease (the “Company” or “Unicycive”), today announced the initial results from the patient reported outcome survey conducted during the UNI-OLC-201 pivotal clinical trial. The positive top-line results from the oxylanthanum carbonate (OLC) trial in patients with hyperphosphatemia who have chronic kidney disease on dialysis were reported on June 25, 2024.
The patient reported outcomes are being evaluated from a satisfaction questionnaire that was a pre-specified exploratory objective of the study. The questionnaire surveyed patients in the UNI-OLC-201 trial to assess characteristics of their current phosphate binder as compared to OLC after switching medications. The questions included patient satisfaction, ease of use, and preferred therapy and were taken at the start and conclusion of the study. In the survey, OLC consistently outperformed the other phosphate binders in all categories:
“We are gratified by the encouraging patient reported findings from our pivotal trial that mirror the better-than-expected topline clinical results that we reported last month,” said, Shalabh Gupta, MD, Chief Executive Officer of Unicycive. “In the design of our pivotal clinical trial for OLC, we believed that it was important to consider the patient perspective and the personal challenges that they face in managing their hyperphosphatemia. Importantly, the results showed that patients preferred OLC greater than 4 to 1 over their prior phosphate binder therapy. Our focus is now directed toward filing our New Drug Application and making OLC available to patients who may benefit from its potential best-in-class profile, if approved.”
Pablo Pergola, MD, PhD, Research Director, Clinical Advancement Center, Renal Associates, P.A., and principal investigator for the UNI-OLC-201 trial, commented, “In this clinical study, our patients stated a clear preference for OLC over their prior phosphate lowering therapies. This positive patient reported experience with OLC is encouraging because hyperphosphatemia outcomes are often negatively impacted by non-adherence to phosphate lowering prescriptions due to side effects and high pill burden. At the end of the study, several of my patients asked not to be put back on their prior phosphate binder.”
Background
Patients screened to enter the trial were taking the following phosphate binder therapies (n=128):
Key Findings
Preferred Therapy: In response to the question: Based on your experience in this clinical trial, do you prefer your current phosphate binder or OLC, 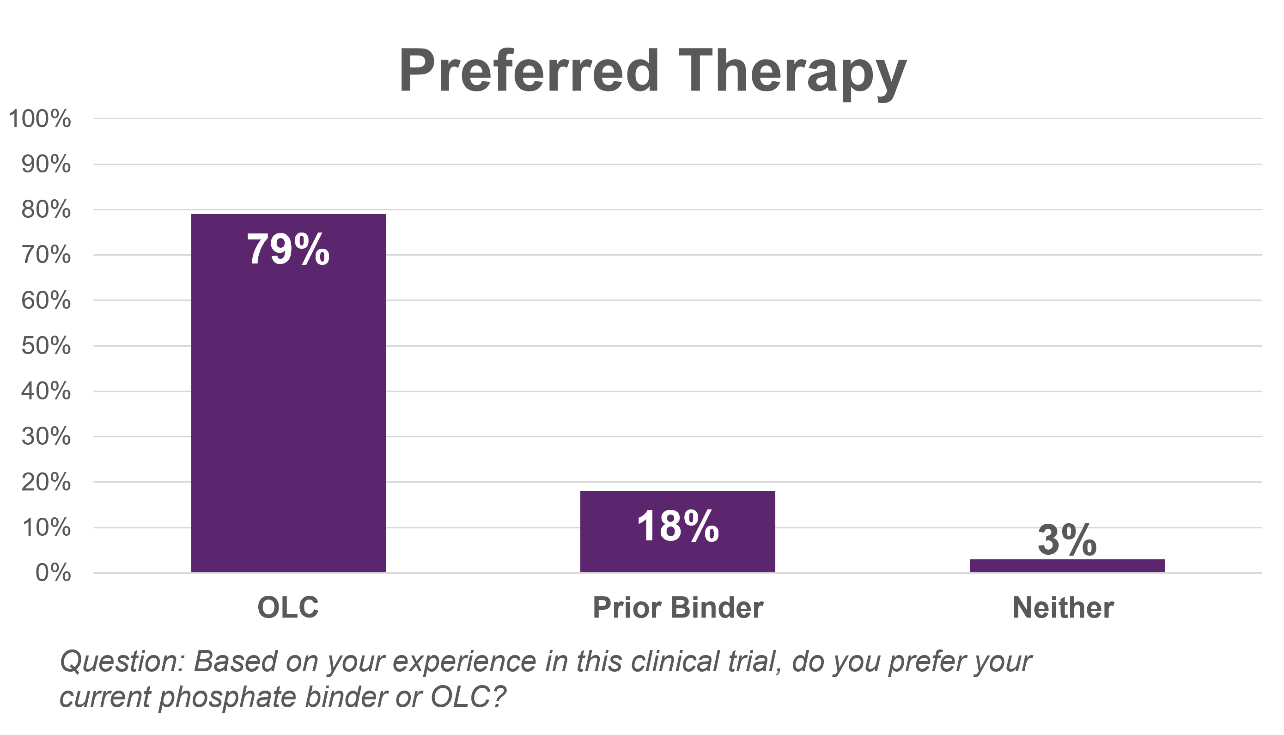
Ease of Use: In the trial, the median patient pill burden on OLC was reduced by half compared to their prior phosphate binder therapy. The pill burden on prior therapy at screening was a median of 6 (mean 6.5) pills per day. On OLC, the pill burden at the end of the study was a median of 3 (mean 3.9) pills per day.
In response to the question: My current phosphate binding medication is easy to take,
Patient Satisfaction: At screening, less than half of the patients in the study agreed with the statement, I am satisfied with my current phosphate binder medication. At the end of the study and after switching to OLC,
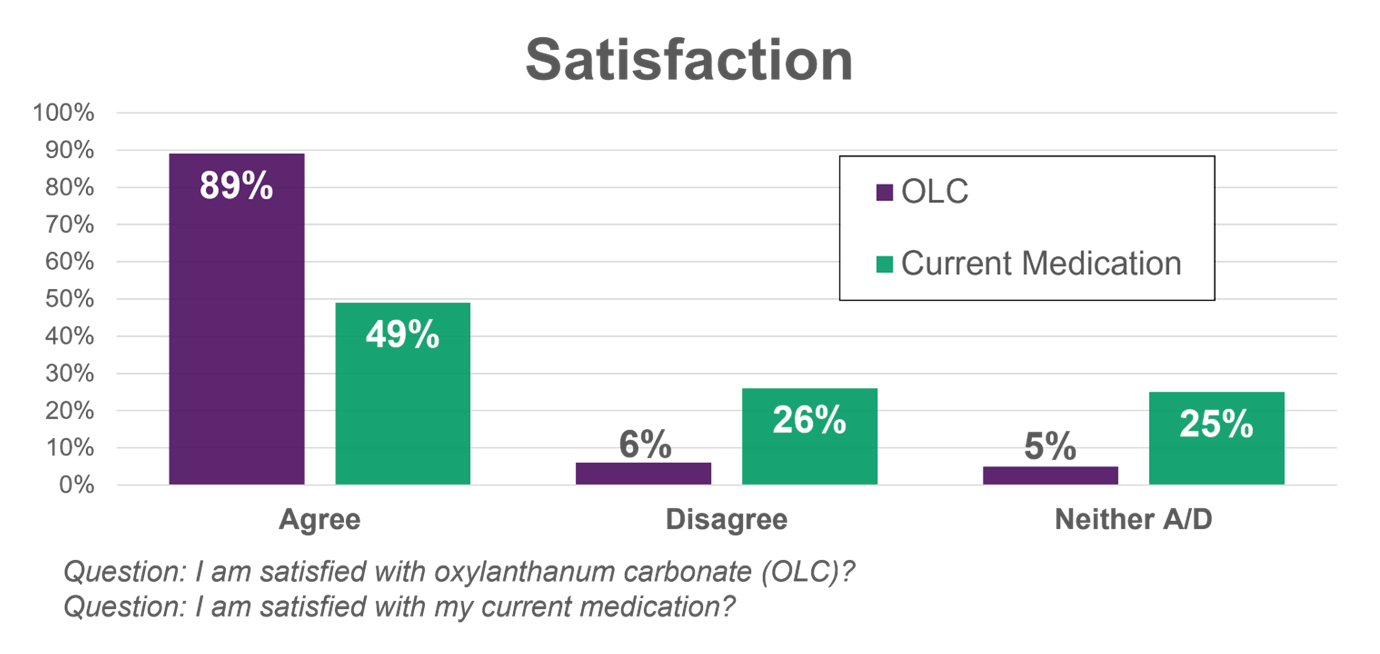
The initial findings from the Oxylanthanum carbonate (OLC) pivotal trial satisfaction questionnaire are preliminary and subject to change based on further detailed analysis. Full survey results are expected to be presented at a future medical conference.
About Oxylanthanum Carbonate (OLC)
Oxylanthanum carbonate is a next-generation lanthanum-based phosphate binding agent utilizing proprietary nanoparticle technology being developed for the treatment of hyperphosphatemia in patients with chronic kidney disease (CKD). OLC has over forty issued and granted patents globally. Its potential best-in-class profile may have meaningful patient adherence benefits over currently available treatment options as it requires a lower pill burden for patients in terms of number and size of pills per dose that are swallowed instead of chewed. Based on a survey conducted in 2022, Nephrologists stated that the greatest unmet need in the treatment of hyperphosphatemia with phosphate binders is a lower pill burden and better patient compliance.1 The global market opportunity for treating hyperphosphatemia is projected to be in excess of
Unicycive is seeking FDA approval of OLC via the 505(b)(2) regulatory pathway. As part of the clinical development program, two clinical studies were conducted in over 100 healthy volunteers. The first study was a dose-ranging Phase I study to determine safety and tolerability. The second study was a randomized, open-label, two-way crossover bioequivalence study to establish pharmacodynamic bioequivalence between OLC and Fosrenol. Based on the results of the bioequivalence study, pharmacodynamic (PD) bioequivalence of OLC to Fosrenol was established. A pivotal clinical trial was also conducted in CKD patients on hemodialysis that achieved the study objective and established favorable tolerability of OLC at clinically effective doses.
About Hyperphosphatemia
Hyperphosphatemia is a serious medical condition that occurs in nearly all patients with End Stage Renal Disease (ESRD). If left untreated, hyperphosphatemia leads to secondary hyperparathyroidism (SHPT), which then results in renal osteodystrophy (a condition similar to osteoporosis and associated with significant bone disease, fractures and bone pain); cardiovascular disease with associated hardening of arteries and atherosclerosis (due to deposition of excess calcium-phosphorus complexes in soft tissue). Importantly, hyperphosphatemia is independently associated with increased mortality for patients with chronic kidney disease on dialysis. Based on available clinical data to date, over
Dialysis patients are already at an increased risk for cardiovascular disease (because of underlying diseases such as diabetes and hypertension), and hyperphosphatemia further exacerbates this. Treatment of hyperphosphatemia is aimed at lowering serum phosphate levels via two means: (1) restricting dietary phosphorus intake; and (2) using, on a daily basis, and with each meal, oral phosphate binding drugs that facilitate fecal elimination of dietary phosphate rather than its absorption from the gastrointestinal tract into the bloodstream.
About Unicycive Therapeutics
Unicycive Therapeutics is a biotechnology company developing novel treatments for kidney diseases. Unicycive’s lead drug candidate, oxylanthanum carbonate (OLC), is a novel investigational phosphate binding agent being developed for the treatment of hyperphosphatemia in chronic kidney disease patients on dialysis. UNI-494 is a patent-protected new chemical entity in clinical development for the treatment of conditions related to acute kidney injury. For more information, please visit Unicycive.com and follow us on LinkedIn, X, and YouTube.
Forward-looking statements
Certain statements in this press release are forward-looking within the meaning of the Private Securities Litigation Reform Act of 1995. These statements may be identified using words such as "anticipate," "believe," "forecast," "estimated" and "intend" or other similar terms or expressions that concern Unicycive's expectations, strategy, plans or intentions. These forward-looking statements are based on Unicycive's current expectations and actual results could differ materially. There are several factors that could cause actual events to differ materially from those indicated by such forward-looking statements. These factors include, but are not limited to, clinical trials involve a lengthy and expensive process with an uncertain outcome, and results of earlier studies and trials may not be predictive of future trial results; our clinical trials may be suspended or discontinued due to unexpected side effects or other safety risks that could preclude approval of our product candidates; risks related to business interruptions, which could seriously harm our financial condition and increase our costs and expenses; dependence on key personnel; substantial competition; uncertainties of patent protection and litigation; dependence upon third parties; and risks related to failure to obtain FDA clearances or approvals and noncompliance with FDA regulations. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors, including: the uncertainties related to market conditions and other factors described more fully in the section entitled ‘Risk Factors’ in Unicycive’s Annual Report on Form 10-K for the year ended December 31, 2023, and other periodic reports filed with the Securities and Exchange Commission. Any forward-looking statements contained in this press release speak only as of the date hereof, and Unicycive specifically disclaims any obligation to update any forward-looking statement, whether as a result of new information, future events or otherwise.
Renvela®️ is a registered trademark of Sanofi.
Phoslo®️ and Velphoro®️ are registered trademarks of Vifor Fresenius
Auryxia®️ is a registered trademark of Akebia Therapeutics
Fosrenol®️ is a registered trademark of Takeda Pharmaceutical Company Limited
1Reason Research, LLC 2022 survey. Results here.
Investor Contact:
ir@unicycive.com
(650) 543-5470
SOURCE: Unicycive Therapeutics, Inc.
Photos accompanying this announcement are available at:
https://www.globenewswire.com/NewsRoom/AttachmentNg/9a233ce4-0844-4e63-b22e-650fa9d140b6
https://www.globenewswire.com/NewsRoom/AttachmentNg/39ea3031-e96f-488a-9212-331b53aa376f
https://www.globenewswire.com/NewsRoom/AttachmentNg/1f4d052e-09f9-4043-bc89-b34cdc87ddcc








