ZygoFix Completes $2 Million Round and Launches A New Clinical Study in Israel
ZygoFix, a Trendlines Group portfolio company, has completed a $2 million funding round for its innovative zLOCK™ spinal implant system, designed as a less invasive alternative to traditional spinal fusion methods. This funding, led by Agriline and supported by additional private investors and the Israel Innovation Authority, will enhance clinical studies, improve the implant system, and facilitate regulatory clearance. The first clinical case in Israel was successfully executed, demonstrating the implant's efficiency in treating severe back pain with minimal risk.
- Successful completion of $2 million funding round to enhance clinical studies and regulatory processes.
- The zLOCK™ implant offers a less invasive solution for spinal fusion, potentially increasing patient safety and satisfaction.
- First clinical case in Israel successfully completed, showcasing the implant's quick application and effectiveness.
- None.
MISGAV, Israel, March 9, 2021 /PRNewswire/ -- ZygoFix, a portfolio company of The Trendlines Group Ltd. (SGX: 42T) (OTCQX: TRNLY), a leading Israel- and Singapore-based investment group focused on high-growth medical and agrifood technologies, announced it has completed
The funding round was led by Agriline (a trust of which Vincent Tchenguiz is a discretionary beneficiary), and Trendlines. Additional private investors and the Israel Innovation Authority also provided funding. The investment will be used to conduct further clinical studies, enhance the system implant and tools, and for regulatory clearance.
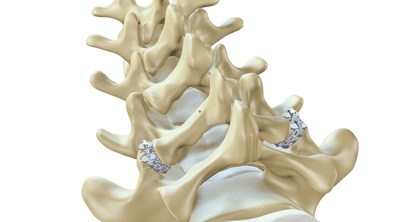
ZygoFix's zLOCK™ is a miniature screwless implant that provides spinal stability and fusion. zLOCK™ is the least invasive, simplest fusion option; it replaces complex screw stabilization, the current gold-standard used in such procedures. The ZygoFix zLOCK implant's patented bendable features enable adjustment to specific joints' shapes during insertion.
ZygoFix's first clinical case in Israel was performed by Dr. Lior Merom at the Rambam Health Care Campus. The surgery included laminotomy and facet stabilization using two zLOCK implants for the treatment of back pain (facet arthritis) and spinal nerve compression (foraminal stenosis). The surgery was completed successfully with placement of the zLOCKs taking less than 30 minutes in total.
Dr. Merom commented: "The simple and very low risk placement of the zLOCKs make it an attractive stabilization solution for suitable indications. We look forward to collecting more data as the study progresses."
This case opens the Israeli clinical study site, following the ongoing study in Hungary which began in 2018. The ongoing study in Hungary has demonstrated meaningful and sustained reduction in pain and increase in quality of life for patients, all with a minimally invasive, simple and short procedure.
ZygoFix CEO Ofer Levy commented, "Our technology rethinks spinal stability. We leverage the natural anatomical structure of the spine and insert the zLOCK implant which locks the motion in the joint.
"We are very satisfied with this first case in Israel as zLOCK adds a new treatment option to the surgeon's toolbox. Its unique ability to perform internal facet fixation with a minimally invasive procedure enables us to treat pathologies in a simple manner, that were too complex and invasive to treat otherwise."
About ZygoFix
ZygoFix was founded in 2017 by Prof. Yizhar Floman and Uri Arnin, experienced serial entrepreneurs in the spine industry (co-founders of ApiFix, acquired in April 2020 by OrthoPediatrics Corp). ZygoFix and ApiFix, both established in the Trendlines Group's incubator, received initial investment from Trendlines and further funding from the Israel Innovation Authority.
Contact information:
Ofer Levy, CEO ZygoFix
ofer@zygofix.com
+972-522-664-981
![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/zygofix-completes-2-million-round-and-launches-a-new-clinical-study-in-israel-301243119.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/zygofix-completes-2-million-round-and-launches-a-new-clinical-study-in-israel-301243119.html
SOURCE ZygoFix
FAQ
What is the purpose of ZygoFix's zLOCK™ implant?
What are the financial implications of ZygoFix's recent funding?
What were the results of the first clinical case in Israel?
How does the zLOCK™ implant compare to traditional spinal fusion techniques?