Ceretrieve Announces Groundbreaking Success in FIH Ischemic Stroke Cases
Ceretrieve announced successful results of its multicenter, single-arm study for its aspiration catheter, aimed at treating acute ischemic stroke (AIS) caused by large vessel occlusion (LVO).
The study involved 20 patients and achieved 100% Complete/Near-Complete Perfusion with the Gen 2 device, far exceeding the 30%-40% rate of current gold-standard devices.
Ceretrieve's device allows single-pass clot removal and full blood flow restoration, ensuring high safety by minimizing the risk of clot fragments moving further into the brain.
The technology demonstrated superior performance compared to existing devices, highlighting its potential to significantly improve post-stroke patient outcomes.
- 100% Complete/Near-Complete Perfusion achieved with Gen 2 device.
- 83% First Pass Complete Perfusion, exceeding current gold-standard rates of 30%-40%.
- Single-pass clot removal and full blood flow restoration.
- High safety standards, reducing the risk of clot fragments moving further into the brain.
- Superior performance compared to existing devices.
- Potential to significantly improve post-stroke patient outcomes.
- Study sample size was to 20 patients.
- Results are preliminary and based on a single-arm study without a control group for comparison.
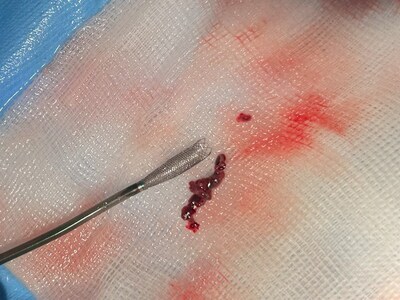
Ceretrieve's aspiration catheter demonstrated clots removal, completely restoring blood flow in a single pass.
YOKNEAM,
Redefining Stroke Treatment
Ceretrieve's mission is to transform stroke care, save lives, minimize disability, and significantly improve post-stroke quality of life. Ceretrieve's aspiration catheter is at the forefront of this mission, delivering aspiration capabilities that are far superior when compared to existing devices, allowing clot(s) removal in a single pass, while fully restoring blood flow. Designed for seamless integration within a conventional 6Fr delivery catheter, Ceretrieve's device offers exceptional trackability and maneuverability, ensuring efficient access to the clot location.
As shown in the study conducted, Ceretrieve's aspiration catheter not only excelled in performance but also ensured the highest safety standards by reducing the risk of releasing clot fragments further into the brain. In the first-in-human (FIH) study
The study included two generations of the device, and the results with the improved Gen 2 device are highly impressive:
Ceretrieve's CEO, Maysa Mustafa: "The promising results of our clinical studies are the culmination of years of hard work and R&D. Our mission to redefine stroke treatment drives everything we do, and these results signify a major leap forward in saving and dramatically improving lives following an ischemic stroke."
"The 'first pass effect' has been shown to improve patient outcomes" says Dr. Shady Jashan (Galilee Medical Center,
Prof. Serder Geyik, MD (Florya Medical Park,
Amir Belson, M.D., Ceretrieve Chair: "With Ceretrieve's advanced technology, we are seeing significant improvements in patient recovery rates. This innovation is setting a new standard in stroke care, offering patients better outcomes and real hope for recovery."
About Ceretrieve
Ceretrieve's vision is to be the doctor's first-choice device in treating ischemic stroke offering complete treatment with superior performance in one device. Ceretrieve was established in 2017 in collaboration with The Trendlines Group (SGX:42T) (OTCQX: TRNLY), a leading investment group based in
Contact:
Maysa Mustafa, CEO
maysa@ceretrieve.com
Photo - https://mma.prnewswire.com/media/2423407/Ceretrieve_case.jpg
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/ceretrieve-announces-groundbreaking-success-in-fih-ischemic-stroke-cases-302156754.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/ceretrieve-announces-groundbreaking-success-in-fih-ischemic-stroke-cases-302156754.html
SOURCE Ceretrieve
FAQ
What were the results of Ceretrieve's study for its aspiration catheter?
How effective is Ceretrieve's aspiration catheter in removing clots?
What is the significance of the first pass effect for Ceretrieve's device?
How does Ceretrieve's device compare to current gold-standard devices?