Rutherrin(R) Increases Efficacy of Chemotherapy for Lung Cancer
Rhea-AI Summary
Theralase Technologies Inc. (TSXV:TLT)(OTCQB:TLTFF) has announced that its lead drug formulation, Rutherrin, significantly enhances the efficacy of Cisplatin in chemotherapy-resistant Non-Small Cell Lung Cancer (NSCLC). In a preclinical study using the Lewis Lung Cancer (LLC1) orthotopic model, mice treated with a combination of Cisplatin and Rutherrin showed a significantly higher survival rate (p0.001) compared to those treated with Cisplatin alone or untreated.This breakthrough could potentially address the issue of chemotherapy drug resistance in cancer treatment. Theralase plans to commence clinical studies for brain cancer, lung cancer, and various blood-based cancers in 2025, pending sufficient capitalization and completion of Good Laboratory Practice toxicology analysis for Rutherrin.
Positive
- Rutherrin significantly enhances Cisplatin efficacy in chemotherapy-resistant NSCLC
- Combination of Cisplatin and Rutherrin showed significantly higher survival rate (p<0.001) in preclinical study
- Potential to address chemotherapy drug resistance in cancer treatment
- Plans to commence clinical studies for multiple cancer types in 2025
Negative
- Clinical studies for Rutherrin are still pending and not yet commenced
- Commencement of clinical studies in 2025 is subject to sufficient capitalization
News Market Reaction
On the day this news was published, TLTFF declined 0.38%, reflecting a mild negative market reaction.
Data tracked by StockTitan Argus on the day of publication.
TORONTO, ON / ACCESSWIRE / August 28, 2024 / Theralase® Technologies Inc. ("Theralase®" or the "Company") (TSXV:TLT)(OTCQB:TLTFF), a clinical stage pharmaceutical company dedicated to the research and development of light, radiation and or drug-activated small molecules and their formulations, intended for the safe and effective destruction of various cancers, bacteria and viruses, is pleased to announce that it's lead drug formulation, Rutherrin®, has demonstrated an ability to provide a significant enhancement of Cisplatin efficacy in chemotherapy resistant Non-Small Cell Lung Cancer ("NSCLC") in a preclinical animal model.
Theralase® recently press released its latest research, using the well-established Lewis Lung Cancer ("LLC1") orthotopic model, representing NSCLC. In this model, mouse lungs are implanted with lung cancer cells, inducing these mice to develop very aggressive, fast growing and metastatic lung tumors.
In further experimentation, mice were treated with Cisplatin (currently one of the most prescribed chemotherapy drugs for lung cancer) in a control group versus Cisplatin combined with Rutherrin® in an active group.
The mice treated with Cisplatin and Rutherrin® demonstrated a significantly higher (p<0.001) enhancement of cisplatin efficacy and a significantly higher (p < 0.001) increase in overall mouse survival. This survival is analogous to a 1 year overall survival in humans and is significant due to the aggressiveness of the LLCI orthotopic model.
As shown in Figure 1, the Kaplan-Meier Curve represents animal survival versus time in days. As can be seen, the combination of Cisplatin with Rutherrin® produced a highly significant increase in overall survival of mice treated versus those who were untreated or treated with Cisplatin only.
Theralase® believes that this preclinical data could be even further improved with the addition of radiation or Metformin to activate Rutherrin®, while resident in NSCLC cells.
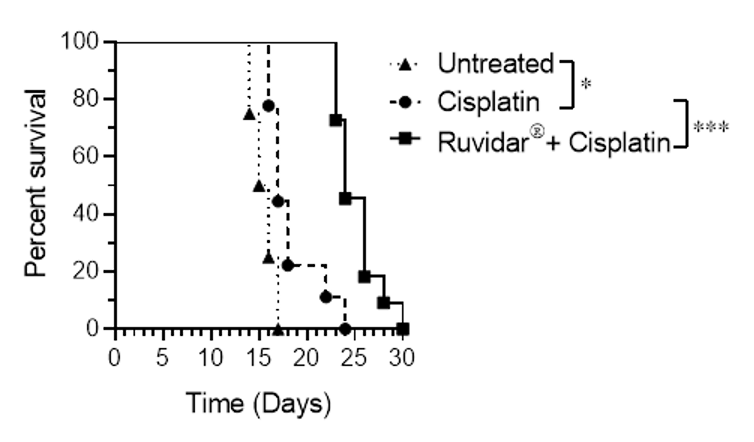
Figure 1: Kaplan-Meier Curve Survival Analysis of Mice After Tumour Inoculation and Treatment with Cisplatin or a Combined Treatment of Rutherrin® and Cisplatin
Dr. Arkady Mandel, M.D., Ph.D., D.Sc., Chief Scientific Officer of Theralase® stated, "Cisplatin-based therapy is one of the most important and highly prescribed chemotherapy treatments for cancer; however, it's efficacy is severely limited by chemotherapy drug resistance, as over time its efficacy decreases. Therefore, it is of significant clinical importance to develop effective synergistic agents to aid in Cisplatin's efficacy. In the latest preclinical study, we have demonstrated the value of using Rutherrin®, as a synergistic agent. The combination of Rutherrin® and Cisplatin exhibited a significant synergistic anti-cancer efficacy and demonstrated significantly longer animal survival (p<0.001). Upon clinical development and commercialization, this preclinical data is very important to the clinical community in the treatment of their lung cancer patients, as it demonstrates how their patients, who do develop chemotherapy resistance, can be treated more effectively with a combination drug therapy, such as Cisplatin and Rutherrin®. These results also suggest an exciting new direction for Theralase® in future drug development to help overcome chemoresistance, which is one of the major clinical challenges in cancer therapy".
Roger DuMoulin-White, B.E.Sc., P.Eng., Pro.Dir., President and Chief Executive Officer of Theralase® stated, "This latest research is extremely important to the clinical community, as pending regulatory approval, it provides an opportunity on how to treat their patient base, who have developed chemotherapeutic resistance to Cisplatin. Pending sufficient capitalization and completion of a Good Laboratory Practice ("GLP") toxicology analysis for Rutherrin®, Theralase® plans to commence clinical studies for brain cancer, lung cancer and various blood-based cancers in 2025. If proven safe and effective in humans, Theralase® hopes to change the paradigm of how patients diagnosed with cancer are treated in the future."
About Cisplatin:
Many chemotherapeutic drugs have been explored and developed for clinical studies. Cisplatin is one of the most potent anti-cancer agents ever developed. It provides clinical effectiveness against a wide spectrum of solid cancers and has been in clinical use for many years; however, many kinds of cancer cells are immune to it or quickly become chemoresistant to it.
About Lung Cancer:
Lung cancer is the leading cause of cancer death worldwide. Most patients die of progressive metastatic disease despite aggressive local and systemic therapies. The survival rate for lung cancer depends on the type, stage and age of the patient, with the overall 5-year survival rate for all types of lung cancer to be about
About Theralase® Technologies Inc.:
Theralase® is a clinical stage pharmaceutical company dedicated to the research and development of light, radiation and/or drug-activated small molecule compounds, their associated drug formulations and the light systems that activate them, with a primary objective of efficacy and a secondary objective of safety in the destruction of various cancers, bacteria and viruses.
Additional information is available at www.theralase.com and www.sedar.com
Neither TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this release.
Forward Looking Statements:
This news release contains "forward-looking statements" within the meaning of applicable Canadian securities laws. Such statements include; but, are not limited to statements regarding the Company's proposed development plans with respect to small molecules and their drug formulations. Forward looking statements may be identified by the use of the words "may, "should", "will", "anticipates", "believes", "plans", "expects", "estimate", "potential for" and similar expressions; including, statements related to the current expectations of Company's management for future research, development and commercialization of the Company's small molecules and their drug formulations, preclinical research, clinical studies and regulatory approvals.
These statements involve significant risks, uncertainties and assumptions; including, the ability of the Company to fund and secure the regulatory approvals to successfully complete various clinical studies in a timely fashion and implement its development plans. Other risks include: the ability of the Company to successfully commercialize its small molecule and drug formulations, the risk that access to sufficient capital to fund the Company's operations may not be available on terms that are commercially favorable to the Company or at all, the risk that the Company's small molecule and drug formulations may not be effective against the diseases tested in its clinical studies, the risk that the Company's fails to comply with the terms of license agreements with third parties and as a result loses the right to use key intellectual property in its business, the Company's ability to protect its intellectual property, the timing and success of submission, acceptance and approval of regulatory filings. Many of these factors that will determine actual results are beyond the Company's ability to control or predict.
Readers should not unduly rely on these forward-looking statements, which are not a guarantee of future performance. There can be no assurance that forward-looking statements will prove to be accurate as such forward-looking statements involve known and unknown risks, uncertainties and other factors which may cause actual results or future events to differ materially from the forward-looking statements.
Although the forward-looking statements contained in the press release are based upon what management currently believes to be reasonable assumptions, the Company cannot assure prospective investors that actual results, performance or achievements will be consistent with these forward-looking statements.
All forward-looking statements are made as of the date hereof and are subject to change. Except as required by law, the Company assumes no obligation to update such statements.
For investor information on the Company, please feel to reach out Investor Inquiries - Theralase Technologies.
For More Information:
1.866.THE.LASE (843-5273)
416.699.LASE (5273)
www.theralase.com
Kristina Hachey, CPA
Chief Financial Officer
X 224
khachey@theralase.com
SOURCE: Theralase Technologies, Inc.
View the original press release on accesswire.com







