Tiziana Life Sciences Announces Positive Results from Ozempic and Nasal Anti-CD3 Combination Study
Tiziana Life Sciences (TLSA) announced positive results from a study combining nasal anti-CD3 (foralumab) with semaglutide (Ozempic). The research, conducted at Brigham and Women's Hospital, demonstrated that the combination improves liver homeostasis and reduces inflammation in diet-induced obesity models. Key findings show synergistic effects in promoting liver health, with the combination therapy resulting in significant reductions in pro-inflammatory cytokines and improvements in liver markers associated with metabolic regulation. The study suggests this novel approach could effectively combat obesity-related inflammation and liver dysfunction.
Tiziana Life Sciences (TLSA) ha annunciato risultati positivi da uno studio che combina l'anti-CD3 nasale (foralumab) con il semaglutide (Ozempic). La ricerca, condotta presso il Brigham and Women's Hospital, ha dimostrato che la combinazione migliora l'omeostasi epatica e riduce l'infiammazione nei modelli di obesità indotta dalla dieta. I risultati chiave mostrano effetti sinergici nella promozione della salute epatica, con la terapia combinata che porta a significative riduzioni delle citochine pro-infiammatorie e miglioramenti nei marcatori epatici associati alla regolazione metabolica. Lo studio suggerisce che questo nuovo approccio potrebbe combattere efficacemente l'infiammazione correlata all'obesità e la disfunzione epatica.
Tiziana Life Sciences (TLSA) anunció resultados positivos de un estudio que combina el anti-CD3 nasal (foralumab) con semaglutida (Ozempic). La investigación, realizada en el Brigham and Women's Hospital, demostró que la combinación mejora la homeostasis hepática y reduce la inflamación en modelos de obesidad inducida por la dieta. Los hallazgos clave muestran efectos sinérgicos en la promoción de la salud del hígado, con la terapia combinada resultando en reducciones significativas de citoquinas proinflamatorias y mejoras en los marcadores hepáticos asociados con la regulación metabólica. El estudio sugiere que este nuevo enfoque podría combatir eficazmente la inflamación relacionada con la obesidad y la disfunción hepática.
티지아나 생명과학 (TLSA)은 코에 분사하는 항-CD3 (포랄루맙)와 세마글루타이드 (오젬픽)를 결합한 연구 결과 긍정적인 결과를 발표했습니다. 브리검 여성 병원에서 실시된 이 연구는 이 조합이 간의 항상성을 개선하고 식이 유도 비만 모델에서 염증을 감소시키는 것을 보여주었습니다. 주요 발견은 간 건강 증진에 있어 상승 효과를 나타내며, 이 조합 요법이 염증을 유발하는 사이토카인을 상당히 줄이고 대사 조절과 관련된 간 지표를 개선하는 결과를 가져왔습니다. 이 연구는 이 새로운 접근 방식이 비만 관련 염증과 간 기능 장애에 효과적으로 대응할 수 있음을 시사합니다.
Tiziana Life Sciences (TLSA) a annoncé des résultats positifs d'une étude combinant l'anti-CD3 nasal (foralumab) avec le semaglutide (Ozempic). La recherche, menée à Brigham and Women's Hospital, a démontré que cette combinaison améliore l'homéostasie hépatique et réduit l'inflammation dans des modèles d'obésité induite par le régime alimentaire. Les résultats clés montrent des effets synergiques pour promouvoir la santé du foie, la thérapie combinée entraînant des réductions significatives des cytokines pro-inflammatoires et des améliorations des marqueurs hépatiques associés à la régulation métabolique. L'étude suggère que cette nouvelle approche pourrait efficacement lutter contre l'inflammation liée à l'obésité et la dysfonction hépatique.
Tiziana Life Sciences (TLSA) hat positive Ergebnisse aus einer Studie bekannt gegeben, die das nasale anti-CD3 (foralumab) mit Semaglutid (Ozempic) kombiniert. Die Forschung, die am Brigham and Women's Hospital durchgeführt wurde, zeigte, dass die Kombination die Leberhomöostase verbessert und die Entzündung in diätinduzierten Adipositasmodellen verringert. Die wichtigsten Ergebnisse zeigen synergistische Effekte zur Förderung der Lebergesundheit, wobei die Kombinationstherapie zu signifikanten Reduktionen der pro-inflammatorischen Zytokine und Verbesserungen der Lebermarker, die mit der Stoffwechselregulation in Verbindung stehen, führt. Die Studie legt nahe, dass dieser neuartige Ansatz effektiv gegen Adipositas-assoziierte Entzündungen und Leberdysfunktion angehen könnte.
- Positive preclinical results showing synergistic effects of foralumab-semaglutide combination
- Demonstrated improvement in liver homeostasis markers
- Significant reduction in inflammation markers in obesity models
- Potential new therapeutic application for foralumab in obesity-related conditions
- Results to preclinical stage, requiring further clinical trials
- Timeline for human trials not specified
- Additional development costs expected for new indication studies
Insights
The combination study of nasal foralumab with semaglutide (Ozempic) shows significant promise in treating obesity-related liver inflammation. The preclinical data demonstrates synergistic effects in promoting liver homeostasis and reducing inflammation markers in diet-induced obesity models.
Key findings reveal that mice treated with the combination therapy showed liver characteristics more similar to healthy lean mice, compared to those treated with semaglutide alone. The dual mechanism - foralumab's immune modulation and semaglutide's metabolic effects - creates a potentially powerful therapeutic approach for treating both obesity and its inflammatory complications.
While these are preclinical results, they lay groundwork for human trials. The market potential is substantial given the growing obesity epidemic and the recent surge in GLP-1 drug popularity. This could position TLSA to capture value in the expanding metabolic disease market, particularly if the combination proves more effective than standalone GLP-1 treatments.
This development could significantly impact TLSA's market position. The collaboration with Ozempic, currently one of the most successful drugs in the market, adds credibility and potential commercial value to TLSA's foralumab program. With Novo Nordisk's GLP-1 drugs generating over
The timing is particularly strategic given the explosive growth in the GLP-1 market and increasing focus on obesity-related conditions. However, investors should note that clinical trials and potential commercialization are still years away and success in animal models doesn't guarantee human efficacy.
NEW YORK, Oct. 30, 2024 (GLOBE NEWSWIRE) -- Tiziana Life Sciences, Ltd. (Nasdaq: TLSA) (“Tiziana” or the “Company”), a biotechnology company developing breakthrough immunomodulation therapies with its lead development candidate, intranasal foralumab, a fully human, anti-CD3 monoclonal antibody, today announced positive results demonstrating the anti-inflammatory potential of our anti-CD3 antibody (foralumab) in combination with semaglutide, a GLP-1 agonist marketed by Novo Nordisk (NYSE: NVO) under the brand names Ozempic and Wegovy. The data show that the combination of nasal anti-CD3 plus semaglutide improves liver homeostasis and reduces inflammation in models of diet-induced obesity (DIO), providing a potential novel approach to combat obesity-related inflammation, and liver inflammation and dysfunction.
Key Highlights:
- Nasal anti-CD3 in combination with semaglutide demonstrates synergistic effects in promoting liver homeostasis in preclinical models of diet-induced obesity.
- The combination significantly reduces inflammation markers, a key factor in obesity-related metabolic disorders.
Figure 1: Nasal anti-CD3 with Low and High Dose semaglutide promote Liver Homeostasis in DIO
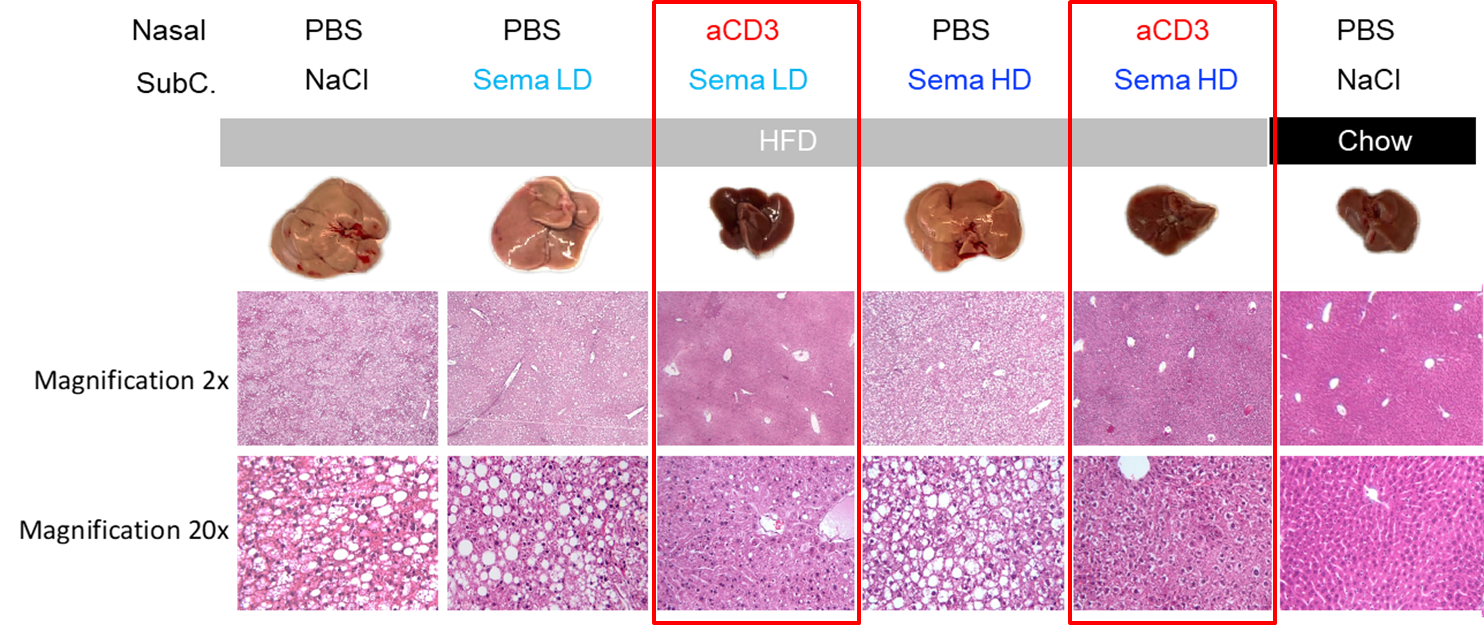
PBS= Placebo, HFD= High Fat Diet, LD= Low Dose, HD= High Dose, Sema= Semaglutide, aCD3= Study Version of Foralumab
In Figure 1, the far-right column shows the explanted liver and histology of that liver at two magnifications for a mouse fed a low-fat chow “normal” diet (“lean mouse”). The dark and smaller liver on the right is a typical healthy liver from a lean mouse. All the mice under the gray bar in the figure were fed a high fat chow (“HFD”) resulting in diet-induced obesity. As the columns outlined by red boxes demonstrate, administration of the combination of nasal anti-CD3 and semaglutide had livers that looked more like the liver from the lean mouse. The HFD mice given low dose or high dose semaglutide alone had enlarged fatty livers that were more similar to the HFD control.
In humans, nasal foralumab modulates immune responses by inducing regulatory-type T cells. Semaglutide is an effective therapy for obesity and Type 2 diabetes, known for its role in enhancing insulin sensitivity and reducing body weight.
This study, conducted by Dr. Howard Weiner and Selma Boulenouar PhD, and a research team at Brigham and Women’s Hospital, Boston, Massachusetts, demonstrates that nasal anti-CD3 in combination with Semaglutide, helps restore liver homeostasis in diet-induced obesity models where liver dysfunction and inflammation are prominent. The combination therapy led to marked reductions in pro-inflammatory cytokines and significant improvements in liver markers associated with metabolic regulation. This suggests a dual benefit in both managing obesity and preventing its associated inflammation-related complications.
"We are excited by the potential of this novel combination. Nasal foralumab’s ability to modulate immune response in humans has always been promising. Now, combining anti-CD3 with semaglutide resulted in additional benefit on liver homeostasis and reduced inflammation in this mouse model. This could pave the way for an entirely new approach in treating obesity-related metabolic disorders," said Dr. Howard L. Weiner, Chairman of Tiziana’s Scientific Advisory Board and co-director of the Ann Romney Center for Neurologic Diseases at Brigham and Women’s Hospital, a founding member of Mass General Brigham healthcare system.
Selma Boulenouar PhD, a leading researcher at Brigham and Women's Hospital, who co-led the study, commented: "The results of this study are very encouraging. Chronic inflammation is a hallmark of obesity and liver diseases. The combination of anti-CD3 with Semaglutide appears to mitigate this inflammatory response and restore normal liver function. These findings underscore the potential for combination therapies in addressing complex metabolic and inflammatory conditions."
Obesity is a growing global health concern, with associated metabolic disorders including insulin resistance, NAFLD, and chronic inflammation contributing to increased morbidity and healthcare costs. The combination of nasal Foralumab for modulating the immune system and semaglutide for weight management offers a novel therapeutic combination that addresses both the metabolic and inflammatory aspects of obesity.
Ivor Elrifi, CEO of Tiziana Life Sciences, commented, "We are excited by these findings, which demonstrate nasal Foralumab’s potential to significantly enhance the therapeutic effects of semaglutide. This combination may present a breakthrough approach to treating not only obesity but also the downstream metabolic complications that are often hard to manage with current therapies. We would like to thank Howard and Selma and the BWH team for their thorough study that has taken many months to complete. We look forward to the full publication of these findings in a peer-reviewed journal."
Tiziana plans to advance these promising preclinical findings into further clinical development, with the goal of initiating in-human clinical trials in the future. This effort is aligned with the Company’s commitment to pioneering innovative solutions for inflammatory and metabolic diseases, leveraging the immunomodulatory properties of Foralumab.
The study at Brigham and Women’s Hospital is part of Tiziana's broader development program for Foralumab, which includes other inflammatory and autoimmune indications. The Company remains dedicated to advancing scientific innovations to address unmet medical needs across diverse therapeutic areas.
About Foralumab
Foralumab, a fully human anti-CD3 monoclonal antibody, is a biological drug candidate that has been shown to stimulate T regulatory cells when dosed intranasally. At present, 10 patients with Non-Active Secondary Progressive Multiple Sclerosis (na-SPMS) have been dosed in an open-label intermediate sized Expanded Access (EA) Program with either an improvement or stability of disease seen within 6 months in all patients. The FDA has recently allowed an additional 20 patients to be enrolled in this EA program. In addition, intranasal foralumab is currently being studied in a Phase 2a, randomized, double-blind, placebo-controlled, multicenter, dose-ranging trial in patients with non-active secondary progressive multiple sclerosis (NCT06292923).
Activated T cells play an important role in the inflammatory process. Foralumab, the only fully human anti-CD3 monoclonal antibody (mAb) currently in clinical development, binds to the T cell receptor and dampens inflammation by modulating T cell function, thereby suppressing effector features in multiple immune cell subsets. This effect has been observed in patients with COVID and with multiple sclerosis, as well as in healthy normal subjects. The non-active SPMS intranasal foralumab Phase 2 trial (NCT06292923) began screening patients in November of 2023. Immunomodulation by nasal anti-CD3 mAb represents a novel avenue for treatment of neuroinflammatory and neurodegenerative human diseases.[1],[2]
About Tiziana Life Sciences
Tiziana Life Sciences is a clinical-stage biopharmaceutical company developing breakthrough therapies using transformational drug delivery technologies to enable alternative routes of immunotherapy. Tiziana’s innovative nasal approach has the potential to provide an improvement in efficacy as well as safety and tolerability compared to intravenous (IV) delivery. Tiziana’s lead candidate, intranasal foralumab, which is the only fully human anti-CD3 mAb currently in clinical development, has demonstrated a favorable safety profile and clinical response in patients in studies to date. Tiziana’s technology for alternative routes of immunotherapy has been patented with several applications pending and is expected to allow for broad pipeline applications.
For more information about Tiziana Life Sciences and its innovative pipeline of therapies, please visit www.tizianalifesciences.com
Forward-Looking Statements
Certain statements made in this announcement are forward-looking statements. These forward-looking statements are not historical facts but rather are based on the Company's current expectations, estimates, and projections about its industry, its beliefs, and assumptions. Words such as 'anticipates,' 'expects,' 'intends,' 'plans,' 'believes,' 'seeks,' 'estimates,' and similar expressions are intended to identify forward-looking statements. These statements are not guarantees of future performance and are subject to known and unknown risks, uncertainties, and other factors, some of which are beyond the Company's control, are difficult to predict, and could cause actual results to differ materially from those expressed or forecasted in the forward-looking statements. The Company cautions security holders and prospective security holders not to place undue reliance on these forward-looking statements, which reflect the view of the Company only as of the date of this announcement. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors, including: the uncertainties related to market conditions and other factors described more fully in the section entitled ‘Risk Factors’ in Tiziana’s Annual Report on Form 20-F for the year ended December 31, 2023, and other periodic reports filed with the Securities and Exchange Commission.The forward-looking statements made in this announcement relate only to events as of the date on which the statements are made. The Company will not undertake any obligation to release publicly any revisions or updates to these forward-looking statements to reflect events, circumstances, or unanticipated events occurring after the date of this announcement except as required by law or by any appropriate regulatory authority.
For further inquiries:
Tiziana Life Sciences Ltd
Paul Spencer, Business Development and Investor Relations
+44 (0) 207 495 2379
email: info@tizianalifesciences.com
[1] https://www.pnas.org/doi/10.1073/pnas.2220272120
[2] https://www.pnas.org/doi/10.1073/pnas.2309221120
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/b407c72c-eb35-4624-a292-a44b5f4af403








