TFF Pharmaceuticals Provides Continued Positive Outcomes from Tacrolimus Inhalation Powder (TFF TAC) Phase 2 Trial for the Prevention of Lung Transplant Rejection
TFF Pharmaceuticals (NASDAQ: TFFP) has reported positive outcomes from its ongoing Phase 2 trial of Tacrolimus Inhalation Powder (TFF TAC) for preventing lung transplant rejection. Key highlights include:
1. Accelerated patient enrollment with 13 patients now in the trial.
2. TFF TAC at ~20% of oral tacrolimus dose prevented acute rejection and achieved >80% of oral trough blood levels.
3. 100% of patients completing 12-week treatment chose to continue in the long-term extension phase.
4. Observed 6.5-fold reduction in abnormally expressed rejection-related gene sets after TFF TAC treatment.
5. No production of donor-specific antibodies (DSA) after TFF TAC treatment.
The company is finalizing the design for the next study in collaboration with clinical investigators and regulatory authorities, with updates expected later this fall.
TFF Pharmaceuticals (NASDAQ: TFFP) ha riportato esiti positivi dal suo studio di Fase 2 in corso sul Tacrolimus Inhalation Powder (TFF TAC) per prevenire il rigetto dei trapianti polmonari. I punti salienti includono:
1. Accelerazione dell'arruolamento dei pazienti con 13 pazienti attualmente nello studio.
2. TFF TAC a circa il 20% della dose orale di tacrolimus ha prevenuto il rigetto acuto e ha raggiunto oltre l'80% dei livelli ematici minimi orali.
3. Il 100% dei pazienti che hanno completato il trattamento di 12 settimane ha scelto di continuare nella fase di estensione a lungo termine.
4. È stata osservata una riduzione di 6,5 volte nei set di geni espressi anormalmente associati al rigetto dopo il trattamento con TFF TAC.
5. Nessuna produzione di anticorpi specifici per il donatore (DSA) dopo il trattamento con TFF TAC.
L'azienda sta finalizzando il design per il prossimo studio in collaborazione con investigatori clinici e autorità regolatorie, con aggiornamenti previsti per questa autunno.
TFF Pharmaceuticals (NASDAQ: TFFP) ha reportado resultados positivos de su ensayo de Fase 2 en curso sobre el Tacrolimus Inhalation Powder (TFF TAC) para prevenir el rechazo de trasplantes de pulmón. Los puntos clave incluyen:
1. Aceleración en la inclusión de pacientes, con 13 pacientes actualmente en el ensayo.
2. TFF TAC a aproximadamente el 20% de la dosis oral de tacrolimus evitó el rechazo agudo y logró más del 80% de los niveles mínimos de sangre orales.
3. El 100% de los pacientes que completaron el tratamiento de 12 semanas optaron por continuar en la fase de extensión a largo plazo.
4. Se observó una reducción de 6.5 veces en los conjuntos de genes relacionados con el rechazo que se expresaron anómalamente después del tratamiento con TFF TAC.
5. No se produjo anticuerpos específicos de donante (DSA) después del tratamiento con TFF TAC.
La empresa está finalizando el diseño del próximo estudio en colaboración con investigadores clínicos y autoridades regulatorias, con actualizaciones esperadas para este otoño.
TFF 제약(TFF Pharmaceuticals, NASDAQ: TFFP)는 폐 이식 거부 반응을 예방하기 위한 타크롤리무스 흡입 분말(TFF TAC)의 진행 중인 2상 시험에서 긍정적인 결과를 보고하였습니다. 주요 사항은 다음과 같습니다:
1. 현재 13명의 환자가 시험에 참여하여 환자 등록이 가속화되었습니다.
2. TFF TAC는 경구 타크롤리무스 용량의 약 20%에서 급성 거부를 예방하고 경구 최저 혈중 농도의 80% 이상을 달성하였습니다.
3. 12주 치료를 완료한 환자의 100%가 장기 연장 단계에 계속 참여하기로 선택하였습니다.
4. TFF TAC 치료 후 거부 반응 관련 유전자 세트에서 비정상적으로 발현된 유전자가 6.5배 감소한 것으로 관찰되었습니다.
5. TFF TAC 치료 후 기증자 특정 항체(DSA)가 생산되지 않았습니다.
회사는 임상 연구자 및 규제 당국과 협력하여 다음 연구 디자인을 마무리 중이며, 이번 가을에 업데이트가 있을 예정입니다.
TFF Pharmaceuticals (NASDAQ: TFFP) a rapporté des résultats positifs de son essai de phase 2 en cours sur la poudre d'inhalation de tacrolimus (TFF TAC) pour prévenir le rejet des greffes pulmonaires. Les points clés incluent :
1. Accélération de l'enrôlement des patients avec 13 patients actuellement dans l'essai.
2. TFF TAC à environ 20 % de la dose de tacrolimus oral a prévenu le rejet aigu et atteint plus de 80 % des niveaux sanguins minimaux oraux.
3. 100 % des patients ayant terminé le traitement de 12 semaines ont choisi de continuer dans la phase d'extension à long terme.
4. Une réduction de 6,5 fois des ensembles de gènes exprimés de manière anormale liés au rejet a été observée après le traitement par TFF TAC.
5. Aucune production d'anticorps spécifiques au donneur (DSA) après le traitement par TFF TAC.
L'entreprise finalise la conception de la prochaine étude en collaboration avec des investigateurs cliniques et des autorités réglementaires, avec des mises à jour prévues pour cet automne.
TFF Pharmaceuticals (NASDAQ: TFFP) hat positive Ergebnisse aus seiner laufenden Phase-2-Studie über Tacrolimus-Inhalationspulver (TFF TAC) zur Verhinderung von Lungentransplantatsabstoßungen berichtet. Zu den Hauptpunkten gehören:
1. Beschleunigte Patientenrekrutierung mit derzeit 13 Patienten in der Studie.
2. TFF TAC in etwa 20% der oralen Tacrolimus-Dosis verhinderte akute Abstoßung und erreichte über 80% der oralen Mindestblutspiegel.
3. 100% der Patienten, die die 12-wöchige Behandlung abgeschlossen haben, entschieden sich, in der langfristigen Verlängerungsphase weiterzumachen.
4. Eine 6,5-fache Reduktion abnormal exprimierter abstoßungsbezogener Gen-Sets nach der Behandlung mit TFF TAC wurde beobachtet.
5. Keine Produktion von spenden-spezifischen Antikörpern (DSA) nach der Behandlung mit TFF TAC.
Das Unternehmen finalisiert das Design der nächsten Studie in Zusammenarbeit mit klinischen Ermittlern und Regulierungsbehörden, mit Aktualisierungen, die später in diesem Herbst erwartet werden.
- Accelerated patient enrollment with 13 patients now in the Phase 2 trial
- TFF TAC at ~20% of oral tacrolimus dose prevented acute rejection and achieved >80% of oral trough blood levels
- 100% of patients (9 out of 9) completing 12-week treatment chose to continue in the long-term extension phase
- 6.5-fold reduction in abnormally expressed rejection-related gene sets after TFF TAC treatment
- No production of donor-specific antibodies (DSA) after TFF TAC treatment in the first 8 patients
- Total patient exposure to TFF TAC therapy has reached 2,063 days (5.65 years)
- Reduced systemic variability of tacrolimus with TFF TAC dosing compared to oral tacrolimus
- One patient experienced signs and symptoms of acute rejection due to a dose of TFF TAC that was too low
Insights
The Phase 2 trial results for TFF Pharmaceuticals' Tacrolimus Inhalation Powder (TFF TAC) show promising outcomes for lung transplant rejection prevention. Key findings include:
- TFF TAC at
~20% of oral tacrolimus dose prevented acute rejection and achieved>80% of oral trough blood levels 100% of patients completing 12-week treatment chose to continue- 6.5-fold reduction in abnormally expressed rejection-related gene sets
- No production of donor-specific antibodies (DSA)
These results suggest TFF TAC could potentially transform immunosuppressive therapy in lung transplants, offering lower systemic drug exposure while maintaining efficacy. The accelerated patient enrollment and long-term patient retention indicate positive reception among trial participants.
TFF Pharmaceuticals' TFF TAC trial results are highly encouraging for the company's market potential. Key business implications include:
- Potential for market disruption in the
$7.5 billion immunosuppressant market - Improved patient compliance due to reduced side effects and dosing frequency
- Possible expansion into other organ transplant markets
- Accelerated enrollment could lead to faster time-to-market
The positive data may attract partnership opportunities or increase acquisition interest from larger pharmaceutical companies. However, investors should note that further clinical trials and regulatory approvals are still needed before commercialization.
As a transplant surgeon, I find the TFF TAC results clinically significant. The ability to prevent rejection with lower systemic exposure is a major advancement. Key points:
- Reduced drug burden could potentially decrease long-term complications like chronic kidney disease
- Maintenance of efficacy with
~20% of oral dose suggests improved therapeutic index - Absence of DSA production indicates adequate systemic immunosuppression
- Gene expression data strongly supports efficacy in preventing rejection
If these results hold in larger trials, TFF TAC could become a preferred option for lung transplant patients, potentially improving long-term outcomes and quality of life. However, long-term safety data will be important for widespread adoption.
Patient enrollment has accelerated, now with 13 patients enrolled in trial
TFF TAC at ~
9 out of 9 (
Observed a 6.5-fold reduction in the number of abnormally expressed rejection-related gene sets after treatment with TFF TAC
No production of donor-specific antibodies (DSA) after treatment with TFF TAC
FORT WORTH, Texas, Aug. 06, 2024 (GLOBE NEWSWIRE) -- TFF Pharmaceuticals, Inc. (NASDAQ: TFFP) (“the Company”), a clinical-stage biopharmaceutical company focused on developing and commercializing innovative drug products based on its patented Thin Film Freezing (TFF) technology platform, today provided an update from the Company’s ongoing Phase 2 trial of Tacrolimus Inhalation Powder (TFF TAC) for the prevention of lung transplant rejection.
“The growing body of data from the TFF TAC Phase 2 study continues to suggest that TFF TAC has transformative potential to advance the field of immunosuppressive therapy to prevent lung transplant rejection,” said Dr. Harlan Weisman, Chief Executive Officer of TFF Pharmaceuticals. “We are finalizing the design of the next study with TFF TAC in close collaboration with our clinical investigators and in communication with regulatory authorities and look forward to providing additional updates on the program including a regulatory update later in the fall.”
Clinical Trial Update
- Patient enrollment has accelerated with 13 patients now enrolled in Phase 2 trial
- TFF TAC at ~
20% of the oral tacrolimus dose prevented acute rejection and achieved >80% of the oral trough blood levels leading to diminished drug burden- No signs or symptoms suggestive of acute rejection
- No use of pulse corticosteroids for treatment of rejection
- No spirometry deterioration suggestive of acute rejection
- No chest x-ray findings suggestive of acute rejection
- No biomarker evidence of acute rejection (gene expression and donor-specific antibody)
- Lower doses and no first pass effect to generate drug metabolites decreases drug burden with TFF TAC compared with oral tacrolimus.
- 9 out of 9 (
100% ) patients who completed the 12-week treatment period with TFF TAC chose to remain on the therapy by proceeding to the long-term extension phase;- 2 patients have been treated for over 1 year, and 6 patients have been treated for more than 6 months;
- Total patient exposure to TFF TAC therapy has now reached 2,063 days, or a total of 5.65 years.
- PK data from the Phase 2 study continue to indicate that TFF TAC dosing results in reduced systemic variability of tacrolimus; the systemic tacrolimus trough to peak concentration swings that occur with oral tacrolimus are not present with TFF TAC, which is predicted to reduce the risk of organ rejection and systemic toxicities such as chronic kidney disease.
- Confirmatory biomarker data from the Phase 2 study also remain positive:
- Updated biomarker data indicate a 6.5-fold reduction in the number of abnormally expressed rejection-related gene sets after 12 weeks of treatment with TFF TAC. These data further suggest TFF TAC has the potential to provide sufficient immunosuppression to prevent rejection
23% to4% reduction (-85% ) in the expression of rejection related genes: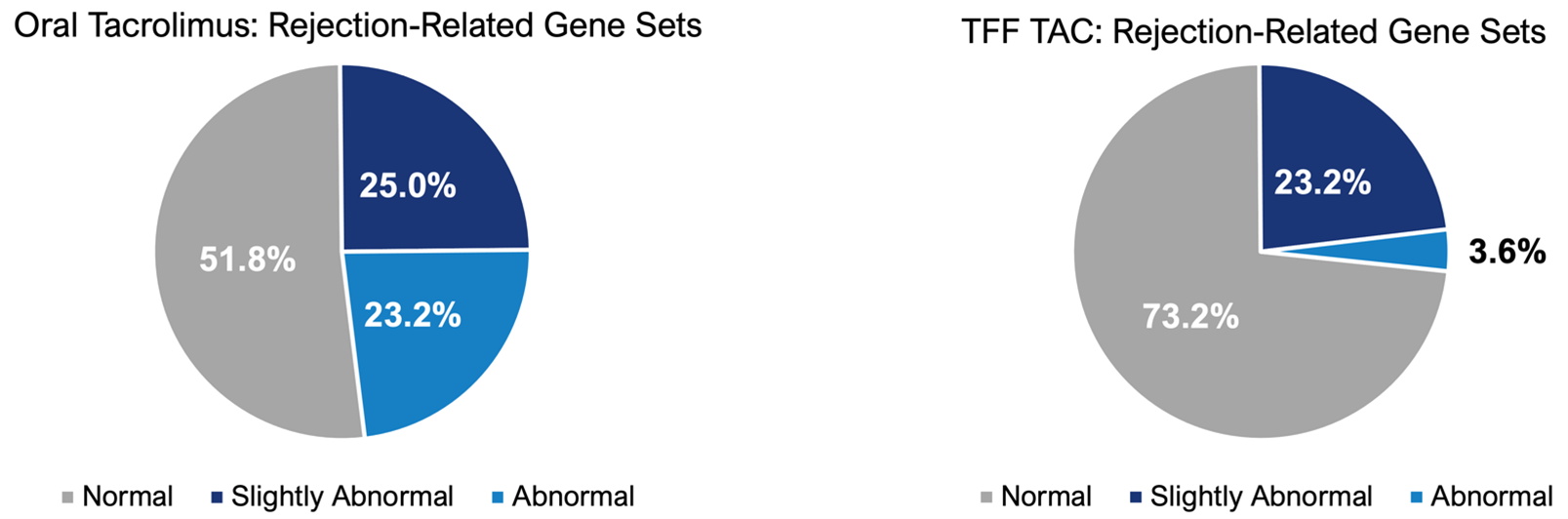
- New biomarker data exploring the presence of donor-specific antibodies (DSA) are now available in the first 8 patients from the study. DSA is known to drive antibody-mediated rejection and is generated when there is insufficient immune suppression systemically allowing the formation of antibodies in the lymph nodes against the transplanted (donor) organ. DSA was negative for the first 8 patients on oral tacrolimus, and DSA remained negative after 12 weeks of treatment with TFF TAC.
- Updated biomarker data indicate a 6.5-fold reduction in the number of abnormally expressed rejection-related gene sets after 12 weeks of treatment with TFF TAC. These data further suggest TFF TAC has the potential to provide sufficient immunosuppression to prevent rejection
- With respect to TFF TAC safety and tolerability, there has been no mortality. The majority of treatment emergent adverse events were Grade 2 (moderate) or lower with no bronchospasm or wheezing reported. Kidney function has been maintained.
- One patient was transitioned to a dose of TFF TAC that was too low, which led to blood trough levels that were >
50% below the protocol-specified minimum. This patient experienced signs and symptoms of acute rejection (but minimal on histopathology). TFF TAC was discontinued as required by the protocol and oral tacrolimus was resumed. The acute rejection episode has resolved.
- TFF is finalizing the design of the next study with TFF TAC in close collaboration with clinical investigators and in communication with regulatory authorities and plans to provide additional updates on the program including a regulatory update later in the fall.
“As the number of patients treated in our Phase 2 study continues to grow, we are highly encouraged by the emerging positive product profile for TFF TAC,” said Dr. Zamaneh Mikhak, Chief Medical Officer of TFF Pharmaceuticals. “For this update, we showed the available new data related to DSA for the first eight patients in the study. This analysis indicates that no patients have developed DSA after transitioning from oral tacrolimus to TFF TAC, suggesting sufficient systemic immune suppression to inhibit production of potentially harmful antibodies despite the modest decrease in blood trough levels. In addition, our updated gene expression data from now 8 patients show a dramatic 6.5-fold reduction in the number of abnormally expressed rejection-related gene sets, suggesting that TFF TAC has the potential to impart sufficient levels of immunosuppression to prevent rejection.”
ABOUT TFF PHARMACEUTICALS’ THIN FILM FREEZING (TFF) TECHNOLOGY
TFF Pharmaceuticals’ proprietary Thin Film Freezing (TFF) technology allows for the transformation of both existing compounds and new chemical entities into dry powder formulations exhibiting unique characteristics and benefits. The TFF process is a particle engineering process designed to generate dry powder particles with advantageous properties for inhalation, as well as parenteral, nasal, oral, topical and ocular routes of administration. The process can be used to engineer powders for direct delivery to the site of need, circumventing challenges of systemic administration and leading to improved bioavailability, faster onset of action, and improved safety and efficacy. The ability to deliver therapies directly to the target organ, such as the lung, allows TFF powders to be administered at lower doses compared to oral drugs, reducing unwanted toxicities and side effects. Laboratory data suggests the aerodynamic properties of the powders created by TFF can deliver as much as
ABOUT TFF PHARMACEUTICALS
TFF Pharmaceuticals, Inc. is a clinical-stage biopharmaceutical company engaging patented rapid freezing technology to develop and transform medicines into potent dry powder formulations for better efficacy, safety, and stability. The company’s versatile TFF technology platform has broad applicability to convert most any drug, including vaccines, small and large molecules, and biologics, into an elegant dry powder highly advantageous for inhalation or for topical delivery to the eyes, nose and skin.
SAFE HARBOR
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Such forward-looking statements in this press release include, but are not limited to, statements by the Company relating to the innovation and commercial potential of the Company’s TFF TAC product candidate to advance the field of immunosuppressive therapy to prevent lung transplant rejection. Forward-looking statements involve known and unknown risks, uncertainties and other factors that could cause actual results to differ materially, including (i) the risk that further data from the Company’s ongoing Phase 2 trial of TFF TAC may not be consistent with the positive preliminary data obtained to date, (ii) the risk that the Company may not be able to obtain additional working capital with which to continue its current operations and clinical trials as and when needed, (iii) success in early phases of pre-clinical and clinical trials do not ensure later clinical trials will be successful; (iv) no drug product incorporating the TFF platform has received FDA pre-market approval or otherwise been incorporated into a commercial drug product, (v) the Company has no current agreements or understandings with any large pharmaceutical companies for the development of a drug product incorporating the TFF Platform, and (vi) those other risks disclosed in the section “Risk Factors” included in the Company’s Quarterly Report on Form 10-Q filed with the SEC on May 14, 2024. The Company cautions readers not to place undue reliance on any forward-looking statements. The Company does not undertake and specifically disclaims any obligation to update or revise such statements to reflect new circumstances or unanticipated events as they occur, except as required by law.
Investor Relations Contact:
Corey Davis, Ph.D.
LifeSci Advisors
(212) 915-2577
cdavis@lifesciadvisors.com
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/69052a47-d65e-4cb7-9528-92212f06cb89








