Tetra Bio-Pharma Announces Start of the REBORN1(C) Clinical Trial
Tetra Bio-Pharma has initiated its first Phase 2 clinical trial, REBORN1, evaluating the inhaled drug QIXLEEF against oral morphine for cancer pain management. Conducted in the U.S. with the Hassman Research Institute, this trial aims to enroll 20 patients experiencing breakthrough cancer pain. QIXLEEF, containing both THC and CBD, seeks to provide a safer alternative to opioids. With cancer pain affecting up to 70% of patients, the potential impact of this drug on pain relief could be significant, indicating a transformative shift in pain management.
- Initiation of the Phase 2 REBORN1 clinical trial for QIXLEEF.
- QIXLEEF aims to provide faster pain relief compared to oral morphine.
- Potential to transform the stagnant pain market by offering a safer alternative.
- None.
Insights
Analyzing...
First ever Phase 2 clinical trial designed to evaluate the effect of cannabis against an opioid treatment
QIXLEEF™ has the potential to transform the pain market
OTTAWA, ON / ACCESSWIRE / May 6, 2021 / Tetra Bio-Pharma Inc. ("Tetra" or the "Company") (TSX:TBP)(OTCQB:TBPMF)(FRA:JAM1), a leader in cannabinoid-derived drug discovery and development today announced the start of the REBORN1© clinical trial. This trial is designed to evaluate the effect of the Company's inhaled proprietary drug formulation, QIXLEEF™, against immediate release oral morphine sulfate on onset of pain relief in people living with cancer. QIXLEEF™ is a botanical drug product with a "fixed ratio" of THC and CBD and is inhaled through a Class 2 medical device vaporizer.
Dr. Guy Chamberland, CEO and CRO of Tetra commented, "Today we recognize an important milestone in advancing the clinical development of this new potential therapeutic for people living with cancer pain. Cancer pain is usually managed with a strong opioid. We believe QIXLEEF™, if proven to be safe and effective, would provide patients with cancer pain a safer treatment option with potentially greater benefits than the current standard of care. QIXLEEF™ may in fact transform the pain market, an area that has been stagnant for many years. Tetra has spent years studying the inhalation of cannabinoids from both synthetic and botanical sources. This research has shown that our investigational new drug, when used with a proprietary medical device, can deliver to patients a reproducible and consistent profile of cannabinoids. The graph below demonstrates the consistency of the inhaled delivery of THC and CBD. We confirm that QIXLEEF™ has arrived in the United States and the trial activities are set to begin."
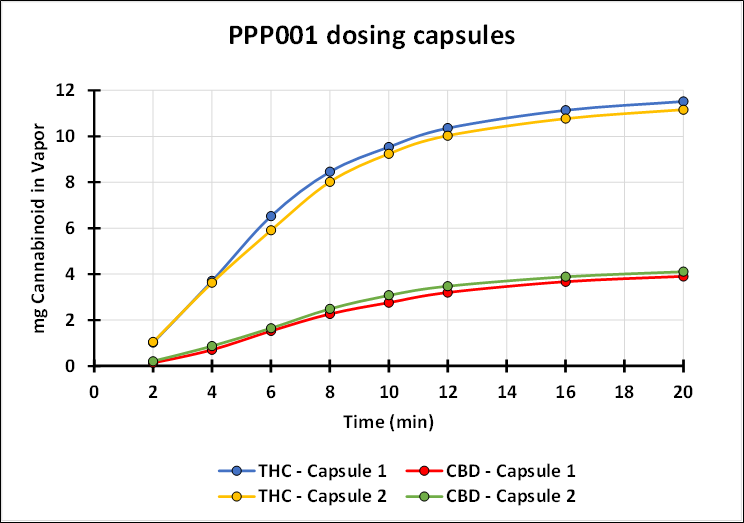
REBORN1© is being conducted in the United States in collaboration with the Hassman Research Institute, a clinical research organization, who will enroll twenty adults living with breakthrough cancer pain (BTcP) and currently taking stable opioid treatment for breakthrough pain. This innovative Phase 2 pilot, proof-of-concept open-label crossover comparison study will assess whether inhaled QIXLEEF™ will control BTcP faster than immediate-release morphine sulfate tablets.
About Breakthrough Cancer Pain
Cancer causes pain in up to
BTcP is defined as "a transient exacerbation of pain that occurs either spontaneously, or in relation to a specific predictable or unpredictable trigger (incident pain), despite relatively stable and adequately controlled background pain" (Davies et al. 2009). Our goal is to decrease and manage this pain and improve the overall quality of life for people with cancer pain.
About the Hassman Research Institute
The Hassman Research Institute (HRI) is a leading clinical research organization that conducts studies in a wide variety of therapeutic areas. HRI's highly trained and experienced staff, including four board certified clinical investigators, is dedicated to maintaining the highest standard of quality results in the trials they conduct.
For more information visit: www.hritrials.com
About Tetra Bio-Pharma
Tetra Bio-Pharma (TSX:TBP)(OTCQB:TBPMF)(FRA:JAM1) is a leader in cannabinoid-derived drug discovery and development with a FDA and a Health Canada cleared clinical program aimed at bringing novel prescription drugs and treatments to patients and their healthcare providers. Our evidence-based scientific approach has enabled us to develop a pipeline of cannabinoid-based drug products for a range of medical conditions, including pain, inflammation, and oncology. With patients at the core of what we do, Tetra Bio-Pharma is focused on providing rigorous scientific validation and safety data required for inclusion into the existing biopharma industry by regulators, physicians and insurance companies.
For more information visit: www.tetrabiopharma.com
Neither the TSX Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Exchange) accepts responsibility for the adequacy or accuracy of this release.
Forward-looking statements
Some statements in this release may contain forward-looking information. All statements, other than of historical fact, that address activities, events or developments that the Company believes, expects or anticipates will or may occur in the future (including, without limitation, statements regarding potential acquisitions and financings) are forward-looking statements. Forward-looking statements are generally identifiable by use of the words "may", "will", "should", "continue", "expect", "anticipate", "estimate", "believe", "intend", "plan" or "project" or the negative of these words or other variations on these words or comparable terminology. Forward-looking statements are subject to a number of risks and uncertainties, many of which are beyond the Company's ability to control or predict, that may cause the actual results of the Company to differ materially from those discussed in the forward-looking statements. Factors that could cause actual results or events to differ materially from current expectations include, among other things, without limitation, the inability of the Company to obtain sufficient financing to execute the Company's business plan; competition; regulation and anticipated and unanticipated costs and delays, the success of the Company's research and development strategies, including the success of this product or any other product, the applicability of the discoveries made therein, the successful and timely completion and uncertainties related to the regulatory process, the timing of clinical trials, the timing and outcomes of regulatory or intellectual property decisions and other risks disclosed in the Company's public disclosure record on file with the relevant securities regulatory authorities. Although the Company has attempted to identify important factors that could cause actual results or events to differ materially from those described in forward-looking statements, there may be other factors that cause results or events not to be as anticipated, estimated or intended. Readers should not place undue reliance on forward-looking statements. The forward-looking statements included in this news release are made as of the date of this news release and the Company does not undertake an obligation to publicly update such forward-looking statements to reflect new information, subsequent events or otherwise unless required by applicable securities legislation.

For further information, please contact Tetra Bio-Pharma Inc.:
Tetra Bio-Pharma Inc.
Ms. Natalie Leroux
Phone: + 1 (833) 977-7575
Email: investors@tetrabiopharma.com
media@tetrabiopharma.com
SOURCE: Tetra Bio-Pharma
View source version on accesswire.com:
https://www.accesswire.com/645077/Tetra-Bio-Pharma-Announces-Start-of-the-REBORN1C-Clinical-Trial







