Stevanato Group Collaborates with Recipharm to Develop and Manufacture Pre-fillable Syringes for Use in a New Soft Mist Inhaler for the Inhalation of Sensitive Biological Products
Stevanato Group S.p.A. (NYSE: STVN) has announced a strategic collaboration with Recipharm, aiming to develop pre-fillable syringes for use in Recipharm’s soft mist inhalers. This partnership leverages Stevanato's 70+ years of manufacturing experience. The Alba® syringes will integrate with Recipharm's Pre-Filled Syringe Inhaler (PFSITM) technology, enhancing drug delivery for sensitive products. The combined offering promises efficient respiratory delivery and improved containment solutions for biopharma companies. This collaboration underscores Stevanato's commitment to supporting drug development from clinical phases to market release.
- Strategic partnership with Recipharm enhances market positioning.
- Utilization of Stevanato's advanced syringe technology improves product efficiency.
- Strengthens capabilities in drug delivery systems, potentially driving revenue growth.
- None.
The new strategic collaboration aims to provide innovative primary packaging to pharmaceutical and biopharmaceutical companies using Recipharm’s proprietary soft mist inhalers.
PIOMBINO DESE,
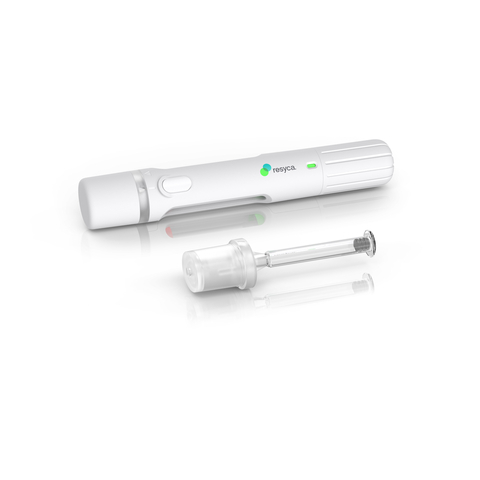
Stevanato Group Collaborates with Recipharm to Develop and Manufacture Pre-fillable Syringes for Use in a New Soft Mist Inhaler for the Inhalation of Sensitive Biological Products (Photo: Business Wire)
This collaboration highlights Stevanato Group’s capabilities as a one-stop shop offering contract drug manufacturing organizations support in their drug development programs, from the clinical phase through to market release. Stevanato Group’s Alba® syringes feature an internal coating based on silicone oil, which is cross-linked with the glass surface, thereby minimizing sub-visible particle release and aiming to ensure superior performance during delivery.
The combination of the Alba® syringe and Recipharm's innovative soft mist inhaler technology delivers sensitive drug products more efficiently to the respiratory airways and provides biopharma companies with a containment solution featuring enhanced stability and safety. With
Recipharm’s soft mist inhaler (PFSI™) is developed, manufactured, and licensed by Resyca® and is supplied as a pre-filled and ready-to-use inhalation device.
Bernhard Muellinger, General Manager and Chief Operational Officer at Resyca® (a joint venture between Recipharm and Medspray), said: “By leveraging
“Biopharma companies are advancing patient care with new, innovative treatments, particularly in biologics and mRNA therapies. These products require specialized, high-performance drug containment systems, like our Alba® syringe platform, together with patient-centric drug delivery devices like the PFSI™. Thanks to our integrated end-to-end capabilities we are able to support our customers at scale with a comprehensive system solution,” said
Forward-Looking Statements
This press release may include forward-looking statements. The words "aims", “will”, "aiming", “accelerate”, “are able”, “creating”, “continue”, and similar expressions (or their negative) identify certain of these forward-looking statements. These forward-looking statements are statements regarding the Company's intentions, beliefs or current expectations concerning, among other things, the investments the Company expects to receive, the expansion of manufacturing capacity, the Company’s plans regarding its presence in the U.S. market, business strategies, the Company’s capacity to meet future market demands and support preparedness for future public health emergencies, and results of operations. The forward-looking statements in this press release are based on numerous assumptions regarding the Company’s present and future business strategies and the environment in which the Company will operate in the future. Forward-looking statements involve inherent known and unknown risks, uncertainties and contingencies because they relate to events and depend on circumstances that may or may not occur in the future and may cause the actual results, performance or achievements of the Company to be materially different from those expressed or implied by such forward-looking statements. Many of these risks and uncertainties relate to factors that are beyond the Company's ability to control or estimate precisely, such as future market conditions, currency fluctuations, the behavior of other market participants, the actions of regulators and other factors such as the Company's ability to continue to obtain financing to meet its liquidity needs, changes in the political, social and regulatory framework in which the Company operates or in economic or technological trends or conditions. Readers should therefore not place undue reliance on these statements, particularly not in connection with any contract or investment decision. Except as required by law, the Company assumes no obligation to update any such forward-looking statements.
About
Founded in 1949,
About Recipharm
Recipharm is a leading
For more information on Recipharm and our services, please visit www.recipharm.com
About Resyca®
Resyca® was founded in 2020 and is a joint venture between Recipharm and Medspray. Resyca® is part of the Recipharm Advanced Delivery Systems business unit. Resyca® develops and manufactures pocket size, user friendly soft-mist inhalation devices, based on standard pre-filled syringe and filled on standard filling lines. As a soft mist development center with Recipharm, Resyca® offers an end-to-end service for soft-mist inhalation products, ranging from early-stage development to commercial manufacture, including filling, labeling, and packaging.
View source version on businesswire.com: https://www.businesswire.com/news/home/20230316005156/en/
Stevanato Group’s media relations:
Investor relations:
Recipharm media contact:
Source:
FAQ
What is the collaboration between Stevanato Group and Recipharm about?
What technology will Stevanato Group provide to Recipharm?
How does this collaboration benefit biopharma companies?







