Starpharma presents compelling data in Prostate Cancer at ESMO
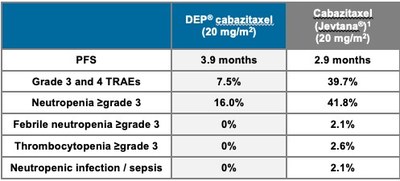
Starpharma has presented promising data on its DEP® cabazitaxel, a nanoparticle formulation of cabazitaxel for advanced prostate cancer (mCRPC), at ESMO 2022. Key findings include a >30% improvement in median progression-free survival (3.9 months vs. 2.9 months) and a 100% response rate among evaluable patients. Compared to standard cabazitaxel, DEP® showed significantly fewer severe treatment-related adverse events (7.5% vs. 39.7%) and no severe hypersensitivity reactions. These results indicate the potential of DEP® cabazitaxel to enhance patient outcomes.
- Improved median progression-free survival by over 30% (3.9 months vs. 2.9 months).
- 100% of evaluable patients showed a response in at least one efficacy measure.
- Lower severe treatment-related adverse events (7.5% vs. 39.7%).
- No severe hypersensitivity reactions or steroid pre-medication required.
- None.
Insights
Analyzing...
MELBOURNE, Australia, Sept. 12, 2022 /PRNewswire/ -- Cabazitaxel (Jevtana, Sanofi) is a market leading chemotherapy for the treatment of advanced prostate cancer (mCRPC). Developed by Australian biotech company Starpharma, DEP® cabazitaxel is a patented, highly water soluble dendrimer nanoparticle version of standard cabazitaxel which has shown, in preclinical and clinical studies, benefits in terms of safety and efficacy.
In a poster presentation at ESMO[1] (European Society of Medical Oncology) by Principal investigator of the Starpharma trial[2], Professor Robert Jones of the Velindre Cancer Centre in Wales, exciting new data on the superior efficacy and lower incidence of key side effects in mCRPC patients was presented.
DEP® cabazitaxel showed multiple potential benefits for patients with mCRPC, including:
- A >
30% improvement in median progression free survival (PFS; time a patient lives without disease progression following treatment) compared to standard cabazitaxel - 3.9 months v 2.9 months respectively 100% of evaluable DEP® cabazitaxel patients achieved a response in at least 1 measure of efficacy (soft tissue disease, prostate specific antigen and/or bone disease)- Lower incidence of severe (Grade 3 or 4) treatment related adverse events (TRAEs) compared to standard cabazitaxel -
7.5% v39.7% respectively - No severe hypersensitivity reactions observed, or steroid pre-medication required, and only 2 patients required prophylactic G-CSF (used after chemotherapy to help white blood cells recover) in contrast to standard cabazitaxel.
Patients enrolled had an average age of 73 and were heavily pre-treated before entering the study (average of 4 other cancer treatment types - 70 cycles/months), in addition to surgery and radiation.
Starpharma CEO, Dr Jackie Fairley, commented: "These results show DEP® cabazitaxel achieved both a longer duration of PFS and fewer severe side effects compared to published data on Jevtana®, illustrating the potential for DEP® cabazitaxel to provide better outcomes for mCRPC patients."
[1] Jones, RH, et al., ESMO 2022 Congress, FPN 1403P. |
[2] Twenty-five patients with mCRPC were enrolled in this cohort across five trial sites in the UK and Australia. Trial participants received DEP® cabazitaxel every 21 days, repeated for up to 12 cycles. |
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/starpharma-presents-compelling-data-in-prostate-cancer-at-esmo-301622463.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/starpharma-presents-compelling-data-in-prostate-cancer-at-esmo-301622463.html
SOURCE Starpharma