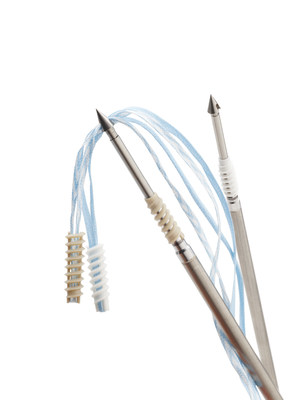
Smith+Nephew launches HEALICOIL™ KNOTLESS Suture Anchor; redefines healing potential for rotator cuff repair
Smith+Nephew has launched the HEALICOIL KNOTLESS Suture Anchor, enhancing its Advanced Healing Solutions portfolio for rotator cuff repairs. This innovative product utilizes a unique open architecture design with REGENESORB Material, promoting faster bone healing and less foreign material in the body. Clinical insights suggest superior healing at the tendon-bone interface. The company aims to redefine healing potential, reinforcing its commitment to patient recovery. Annual sales reached $5.1 billion in 2019, with a strong presence in over 100 countries.
- Launch of HEALICOIL KNOTLESS Suture Anchor enhances Advanced Healing Solutions for rotator cuff repair.
- Unique open architecture promotes faster healing by allowing bone marrow access, leading to improved outcomes.
- REGENESORB Material supports bone integration within 24 months.
- Strong market presence with annual sales of $5.1 billion in 2019.
- None.
Insights
Analyzing...
LONDON, Sept. 9, 2020 /PRNewswire/ -- Smith+Nephew (LSE:SN, NYSE:SNN), the global medical technology business, announces the launch of the HEALICOIL KNOTLESS Suture Anchor, expanding its revolutionary line of Advanced Healing Solutions for rotator cuff repair. This portfolio includes the innovative REGENETEN™ Bioinductive Implant and novel REGENESORB™ Material.
The unique open architecture design of the HEALICOIL KNOTLESS Suture Anchor coupled with REGENESORB Material is designed to facilitate a jump start in bone healing and formation.
With the most open architecture design among leading competitors1* and with less foreign material entering the body1, the repair site can be accessed by bone marrow and associated stem cells leading to healing and better bone ingrowth.2,3
The advanced biocomposite REGENESORB Material encourages the implant to be absorbed and replaced by bone within 24 months.4-6
"The real advantage of the [HEALICOIL] open architecture is that the marrow elements from the bone can reach the bone-tendon interface to promote healing where it is most needed" commented Ian Lo, MD FRCS(C), Assistant Professor, University of Calgary. "We have seen that the anchor leads to more robust healing of the tendon to the bone."
Smith+Nephew's Advanced Healing Solutions for rotator cuff repair is a suite of disruptive technologies that together create an environment designed to enhance the body's biological response to healing.7-11
"We are fully committed to providing our customers with world-class, comprehensive procedural solutions that help not just fix their patients but truly heal them so they can get back to living life unlimited as quickly as possible," said Scott Schaffner, General Manager and Senior Vice President, Sports Medicine, Smith+Nephew. "The launch of HEALICOIL KNOTLESS as part of our Advanced Healing Solutions portfolio is further reinforcement of our commitment to redefine the body's healing potential."
References
- Clark TR, Guerrero EM, Song A, O'Brien MJ, Savoie FH. Do vented suture anchors make a difference in rotator cuff healing. Ann Sports Med Res. 2016;3:1068.
- Vonhoegen J, John D, Hägermann C. Osteoconductive resorption characteristics of a novel biocomposite suture anchor material in rotator cuff repair. Orthop Traumatol Surg Res. 2019;14(1):12.
- Smith + Nephew 2010. Micro-CT and histological evaluation of specimens from resorbable screw study (RS-II / OM1-08) 24-month post-implantation. Internal Report WRP-TE045-700-08.
- Smith + Nephew 2016. HEALICOIL REGENESORB Suture Anchor – a study to assess implant replacement by bone over a 2 year period. NCS248.
- Bokor DJ, Sonnabend D, Deady L, et al. Evidence of healing of partial-thickness rotator cuff tears following arthroscopic augmentation with a collagen implant: a 2-year MRI follow-up. Muscles, ligaments and tendons journal. 2016;6(1):16-25.
- Schlegel TF, Abrams JS, Bushnell BD, Brock JL, Ho CP. Radiologic and clinical evaluation of a bioabsorbable collagen implant to treat partial-thickness tears: a prospective multicenter study. J Shoulder Elbow Surg. 2018 27(2):242-251.
- Van Kampen C, Arnoczky S, Parks P, et al. Tissue-engineered augmentation of a rotator cuff tendon using a reconstituted collagen scaffold: a histological evaluation in sheep. Muscles Ligaments Tendons J. 2013;3(3):229-235.
- Arnoczky SP, Bishai SK, Schofield B, et al. Histologic Evaluation of Biopsy Specimens Obtained After Rotator Cuff Repair Augmented With a Highly Porous Collagen Implant. Arthroscopy. 2017;33(2):278-283
- McElvany MD, McGoldrick E, Gee AO, Neradilek MB, Matsen FA, 3rd. Rotator cuff repair: published evidence on factors associated with repair integrity and clinical outcome. Am J Sports Med. 2015;43(2):491-500.
About Smith+Nephew
Smith+Nephew is a portfolio medical technology business that exists to restore people's bodies and their self-belief by using technology to take the limits off living. We call this purpose 'Life Unlimited'. Our 17,500+ employees deliver this mission every day, making a difference to patients' lives through the excellence of our product portfolio, and the invention and application of new technologies across our three global franchises of Orthopaedics, Advanced Wound Management and Sports Medicine & ENT.
Founded in Hull, UK, in 1856, we now operate in more than 100 countries, and generated annual sales of
For more information about Smith+Nephew, please visit www.smith-nephew.com and follow us on Twitter, LinkedIn, Instagram or Facebook.
Forward-looking Statements
This document may contain forward-looking statements that may or may not prove accurate. For example, statements regarding expected revenue growth and trading margins, market trends and our product pipeline are forward-looking statements. Phrases such as "aim", "plan", "intend", "anticipate", "well-placed", "believe", "estimate", "expect", "target", "consider" and similar expressions are generally intended to identify forward-looking statements. Forward-looking statements involve known and unknown risks, uncertainties and other important factors that could cause actual results to differ materially from what is expressed or implied by the statements. For Smith+Nephew, these factors include: risks related to the impact of COVID-19, such as the depth and longevity of its impact, government actions and other restrictive measures taken in response, material delays and cancellations of elective procedures, reduced procedure capacity at medical facilities, restricted access for sales representatives to medical facilities, or our ability to execute business continuity plans as a result of COVID-19; economic and financial conditions in the markets we serve, especially those affecting health care providers, payers and customers (including, without limitation, as a result of COVID-19); price levels for established and innovative medical devices; developments in medical technology; regulatory approvals, reimbursement decisions or other government actions; product defects or recalls or other problems with quality management systems or failure to comply with related regulations; litigation relating to patent or other claims; legal compliance risks and related investigative, remedial or enforcement actions; disruption to our supply chain or operations or those of our suppliers (including, without limitation, as a result of COVID-19); competition for qualified personnel; strategic actions, including acquisitions and dispositions, our success in performing due diligence, valuing and integrating acquired businesses; disruption that may result from transactions or other changes we make in our business plans or organisation to adapt to market developments; and numerous other matters that affect us or our markets, including those of a political, economic, business, competitive or reputational nature. Please refer to the documents that Smith+Nephew has filed with the U.S. Securities and Exchange Commission under the U.S. Securities Exchange Act of 1934, as amended, including Smith+Nephew's most recent annual report on Form 20-F, for a discussion of certain of these factors. Any forward-looking statement is based on information available to Smith+Nephew as of the date of the statement. All written or oral forward-looking statements attributable to Smith+Nephew are qualified by this caution. Smith+Nephew does not undertake any obligation to update or revise any forward-looking statement to reflect any change in circumstances or in Smith+Nephew's expectations.
™ Trademark of Smith+Nephew. Certain marks registered US Patent and Trademark Office.
![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/smithnephew-launches-healicoil-knotless-suture-anchor-redefines-healing-potential-for-rotator-cuff-repair-301126181.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/smithnephew-launches-healicoil-knotless-suture-anchor-redefines-healing-potential-for-rotator-cuff-repair-301126181.html
SOURCE Smith & Nephew plc