Smith+Nephew announces commercial launch of its AETOS™ Shoulder System for anatomic and reverse shoulder replacement at AAOS 2024 Annual Meeting
- None.
- None.
Insights
The commercial availability of Smith+Nephew's AETOS Shoulder System, coupled with its 510(k) clearance for use with ATLASPLAN 3D Planning Software and Patient Specific Instrumentation, marks a significant advancement in the field of orthopedic surgery, specifically total shoulder arthroplasty (TSA). The integration of 3D planning software into TSA procedures is poised to enhance pre-operative planning, potentially improving surgical outcomes and reducing recovery times. The system's Meta Stem technology, designed for stability and bone preservation, addresses key concerns in TSA by aiming to maintain patient anatomy and minimize arthritic pain.
From a business perspective, the expansion of Smith+Nephew's Upper Extremity portfolio with this system could bolster its market position in the rapidly growing orthopedics segment, projected to reach 250,000 procedures by 2025 in the US alone. The company's collaboration with Materialise NV, leveraging their 30-year experience in personalized medical solutions, further strengthens the product's credibility and could drive adoption rates. For investors, the impact of this launch on Smith+Nephew's revenue growth and market share will be critical to monitor, especially as it relates to their Sports Medicine shoulder repair solutions.
The AETOS Shoulder System's design, which includes metaphyseal fixation and an inlay collar, suggests a focus on stability and bone preservation. These features are particularly relevant in TSA, where the goal is to restore shoulder mobility and reduce pain while minimizing the risk of implant loosening or failure. The system's intraoperative flexibility and streamlined instrumentation could significantly improve the surgical experience, potentially reducing operative times and improving efficiency, especially in Ambulatory Service Center (ASC) environments where sterilization and space are at a premium.
As an orthopedic surgeon, the ability to customize surgical plans for each patient using 3D planning software and patient-specific instrumentation could translate to more predictable surgical outcomes. The potential for improved accuracy in implant placement with the aid of the 3D-printed glenoid guide may also reduce the likelihood of complications, contributing to better patient satisfaction and potentially lower healthcare costs due to fewer revision surgeries.
Smith+Nephew's strategic move to release the AETOS Shoulder System in the US market aligns with the growing demand for advanced medical technologies in orthopedic surgeries. The company's emphasis on developing a comprehensive portfolio for upper extremity care positions it to capitalize on current industry trends, such as the shift towards outpatient surgical settings like ASCs. Given that the system is designed to simplify operating room flow and includes fewer instruments, it may appeal to healthcare providers looking to optimize their resources and reduce costs.
Moreover, the partnership with Materialise NV to incorporate 3D planning software and patient-specific instrumentation reflects a broader industry shift towards personalized healthcare. This trend is likely to be well-received by the market, as personalized solutions often lead to better patient outcomes. The success of the AETOS Shoulder System could serve as a bellwether for Smith+Nephew's innovation trajectory and its potential to disrupt the orthopedic space, with implications for market dynamics and competitive positioning.
Smith+Nephew (LSE:SN, NYSE:SNN), the global medical technology company, today announces full commercial availability of its AETOS Shoulder System in the US, along with 510(k) clearance for its use with ATLASPLAN◊ 3D Planning Software and Patient Specific Instrumentation for total shoulder arthroplasty. Total shoulder arthroplasty is one of the fastest growing segments in Orthopaedics with an estimated 250,000 procedures in the US by 2025.1
Developed to restore patients’ range-of-motion2-5 and help minimize arthritic shoulder pain, the AETOS Shoulder System features the Meta Stem which is designed for stability with metaphyseal fixation and an inlay collar, bone preservation, and to maintain patient anatomy.2,6,7
“After using the system for the past several months, I can say that the AETOS Shoulder System is a game changer,” said Dr. Charles Jobin, Orthopaedic Surgeon at Columbia University Irving Medical Center. “With the stability of its Meta Stem, the flexibility the system provides and streamlined instrumentation, it is a great solution not only benefitting patients but surgeons alike.”
Indicated for both anatomic and reverse total shoulder arthroplasty, the AETOS Shoulder System offers a compact yet comprehensive portfolio of solutions that enhance the surgical experience by enabling intraoperative flexibility. With fewer steps for conversion*8 and fewer instruments for primary anatomic and reverse,*8 the system is designed to simplify the operating room flow.8 This also allows for an efficient solution, particularly of value in an Ambulatory Service Center (ASC) environment where sterilization and space limitations may be of concern.
“I’m thrilled with the stem fixation of the implant that the AETOS Shoulder System provides and believe it will be a noticeable, positive improvement for my patients,” said Dr. Matthew Ramsey, Shoulder and Elbow Specialist at Rothman Orthopaedics. “I’m also very pleased with the instrumentation - especially the glenoid reamer being so low profile and easy to use.”
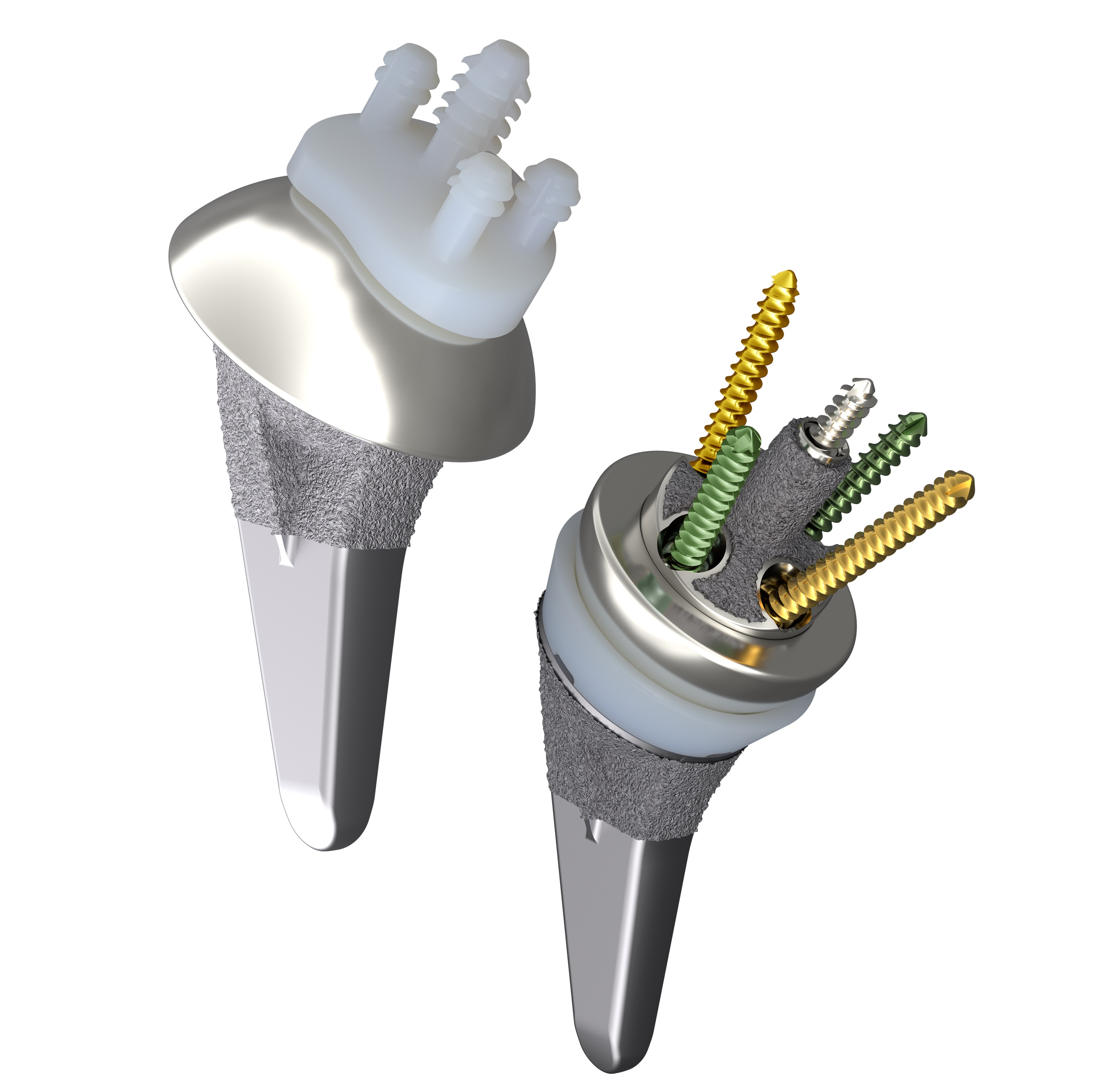
Smith+Nephew's AETOS Shoulder System
ATLASPLAN 3D Planning Software and Patient Specific Instrumentation enables pre-operative case planning for total shoulder arthroplasty, and was developed in partnership with Materialise NV, who has over 30 years of experience revolutionizing personalized solutions. It features:
- A user-friendly online web-based planner for access from any computer or tablet.
- Fast turnaround time from image upload to planning.
- An optional 3D-printed glenoid guide, designed with the patented coracoid clip for stability, provides a reliable and accurate method to execute the surgical plan through conventional surgical techniques.9
The AETOS Shoulder System received 510(k) clearance from the United States Food and Drug Administration in June of 2023. It is the latest addition to Smith+Nephew’s expanding Upper Extremity portfolio and complements our market-leading Sports Medicine shoulder repair solutions to enable biological healing.
To learn more please visit www.smith-nephew.com/aetos or visit the Smith+Nephew booth (#5469) at the American Academy of Orthopaedic Surgeons 2024 Annual Meeting from February 12-16 in San Francisco, CA.
- ends -
Enquiries
| Media David Snyder +1 978-749-1440 Smith+Nephew | |
References
- SmartTrak Report, 2023
- Smith+Nephew 2023. AETOS Inlay Design Features. Internal Report. ER-04-0990-0017.
- Arenas-Miquelez A, Murphy R, Rosa A, Caironi D, Zumstein M. Impact of humeral and glenoid component variations on range of motion in reverse geometry total shoulder arthroplasty. A standardised computer model study. (8214). Swiss Medical Weekly. 2020;150(SUPPL 244):2S.
- Kalouche I, Sevivas N, Wahegaonker A, Sauzieres P, Katz D, Valenti P. Reverse shoulder arthroplasty: Does reduced medialisation improve radiological and clinical results? Acta Orthopaedica Belgica. 2009;75(2):158-166.
- Lädermann A, Tay E, Collin P, et al. Effect of critical shoulder angle, glenoid lateralization, and humeral inclination on range of movement in reverse shoulder arthroplasty. Bone Joint Res. 2019;8(8):378-386.
- Smith+Nephew 2023. AETOS Anatomic Humeral Head Design. Internal Report. ER-04-0990-0019.
- Harmer L, Throckmorton T, Sperling JW. Total shoulder arthroplasty: are the humeral components getting shorter? Curr Rev Musculoskelet Med. 2016;9(1):17-22.
- Smith+Nephew 2023. AETOS Instruments & Trays. Internal Report. ER-04-0990-0020.
- Materialise 3D Shoulder Planner & Guides. Materialise nv L-103300-01
*Compared to a competitive shoulder system
About Smith+Nephew
Smith+Nephew is a portfolio medical technology company focused on the repair, regeneration and replacement of soft and hard tissue. We exist to restore people’s bodies and their self-belief by using technology to take the limits off living. We call this purpose ‘Life Unlimited’. Our 19,000 employees deliver this mission every day, making a difference to patients’ lives through the excellence of our product portfolio, and the invention and application of new technologies across our three global business units of Orthopaedics, Sports Medicine & ENT and Advanced Wound Management.
Founded in Hull, UK, in 1856, we now operate in more than 100 countries, and generated annual sales of
For more information about Smith+Nephew, please visit www.smith-nephew.com and follow us on X, LinkedIn, Instagram or Facebook.
Forward-looking Statements
This document may contain forward-looking statements that may or may not prove accurate. For example, statements regarding expected revenue growth and trading margins, market trends and our product pipeline are forward-looking statements. Phrases such as "aim", "plan", "intend", "anticipate", "well-placed", "believe", "estimate", "expect", "target", "consider" and similar expressions are generally intended to identify forward-looking statements. Forward-looking statements involve known and unknown risks, uncertainties and other important factors that could cause actual results to differ materially from what is expressed or implied by the statements. For Smith+Nephew, these factors include: risks related to the impact of Covid, such as the depth and longevity of its impact, government actions and other restrictive measures taken in response, material delays and cancellations of elective procedures, reduced procedure capacity at medical facilities, restricted access for sales representatives to medical facilities, or our ability to execute business continuity plans as a result of Covid; economic and financial conditions in the markets we serve, especially those affecting healthcare providers, payers and customers (including, without limitation, as a result of Covid); price levels for established and innovative medical devices; developments in medical technology; regulatory approvals, reimbursement decisions or other government actions; product defects or recalls or other problems with quality management systems or failure to comply with related regulations; litigation relating to patent or other claims; legal and financial compliance risks and related investigative, remedial or enforcement actions; disruption to our supply chain or operations or those of our suppliers (including, without limitation, as a result of Covid); competition for qualified personnel; strategic actions, including acquisitions and dispositions, our success in performing due diligence, valuing and integrating acquired businesses; disruption that may result from transactions or other changes we make in our business plans or organisation to adapt to market developments; relationships with healthcare professionals; reliance on information technology and cybersecurity; disruptions due to natural disasters, weather and climate change related events; changes in customer and other stakeholder sustainability expectations; changes in taxation regulations; effects of foreign exchange volatility; and numerous other matters that affect us or our markets, including those of a political, economic, business, competitive or reputational nature. Please refer to the documents that Smith+Nephew has filed with the U.S. Securities and Exchange Commission under the U.S. Securities Exchange Act of 1934, as amended, including Smith+Nephew's most recent annual report on Form 20-F, which is available on the SEC’s website at www. sec.gov, for a discussion of certain of these factors. Any forward-looking statement is based on information available to Smith+Nephew as of the date of the statement. All written or oral forward-looking statements attributable to Smith+Nephew are qualified by this caution. Smith+Nephew does not undertake any obligation to update or revise any forward-looking statement to reflect any change in circumstances or in Smith+Nephew's expectations.
◊ Trademark of Smith+Nephew. Certain marks registered in US Patent and Trademark Office.








