Imperial College London Study Shows Groundbreaking Final Results for SHL Telemedicine’s SmartHeart® in Post-ACS Patient Care
- Groundbreaking results from the TELE-ACS Trial showcase SHL's SmartHeart® technology reducing hospital readmissions by 76% and ED visits by 41%.
- Significant decreases in unplanned revascularizations and patient-reported symptoms were observed in the study.
- The trial, involving 337 participants, sets a new standard in telemedicine for post-ACS patient management.
- Results were presented at ACC 24 and published in JACC, highlighting the impact of SHL's telemedicine solutions on global patient care.
- None.
Insights
The recent findings from the Imperial College London TELE-ACS Trial represent a significant milestone in the telemedicine industry, particularly in the field of remote cardiac care. The substantial reduction in hospital readmissions and emergency department visits is a testament to the efficacy of SHL's SmartHeart® technology. From a medical research perspective, these results suggest a paradigm shift in post-acute coronary syndrome (ACS) patient management.
Traditionally, post-MI patients face a high risk of readmission, often due to inadequate follow-up care and poor management of symptoms. The integration of telemedicine, as evidenced by the trial, not only improves patient monitoring but also enhances the timeliness and personalization of care. This is expected to lead to better patient outcomes, as indicated by the reported symptom reduction. Furthermore, the decrease in unplanned coronary revascularizations highlights the potential for telemedicine to preemptively address complications, thereby reducing the burden on healthcare systems.
Another aspect worth noting is the cost implications for healthcare providers. The shift from in-person to remote monitoring can result in significant cost savings by decreasing the need for hospital resources and potentially reducing the length of in-patient stays. For insurance companies and healthcare payers, these findings could influence reimbursement policies and accelerate the adoption of telemedicine services.
The economic impact of the TELE-ACS Trial's results cannot be overstated. A 76% reduction in hospital readmissions and a 41% decrease in emergency department visits have profound implications for the healthcare economy. These figures suggest a substantial decrease in healthcare utilization, which translates to lower healthcare costs for both providers and patients.
For hospitals, the reduction in readmissions may lead to better allocation of resources and increased availability of beds for other patients, which is important in times of high demand. However, it's important to consider that a shift towards telemedicine could also result in a reallocation of workforce and potential job restructuring within hospital settings.
From a broader economic perspective, the improved management of post-MI patients through telemedicine could lead to a healthier workforce and reduced absenteeism due to health-related issues. In the long run, this could contribute to increased productivity and economic growth. The key challenge will be ensuring equitable access to telemedicine technologies to maximize these potential economic benefits across different socioeconomic groups.
In light of the TELE-ACS Trial results, the market potential for SHL's SmartHeart® and similar telemedicine solutions appears robust. The reported outcomes are likely to resonate with healthcare providers and patients alike, signaling a growing market for remote cardiac care technologies. The successful trial outcomes serve as a powerful marketing tool for SHL Telemedicine, potentially increasing its market share and stock value.
Investors should monitor the adoption rate of SHL's technology following these results. A surge in demand is plausible, as healthcare providers seek to replicate the trial's success. Additionally, competitors in the telemedicine space may accelerate their research and development efforts to produce comparable or superior solutions, sparking innovation and potentially leading to a more competitive market.
It is also vital to consider regulatory aspects. The acceptance of telemedicine by regulatory bodies, influenced by such clinical trial outcomes, could lead to more favorable policies and increased funding for telehealth initiatives. This, in turn, could further drive the market growth and expansion of companies like SHL Telemedicine.
Groundbreaking Study reveals a
TEL AVIV & ZURICH &
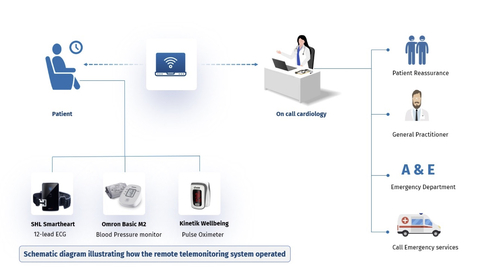
Photo: Visual Abstract from TELE-ACS trial presentation at ACC24 (Graphic: Business Wire)
Key Findings from the Study:
-
A
76% reduction in the likelihood of hospital readmission within six months for patients using telemedicine. -
A
41% decrease in the likelihood of attending the emergency department (ED) compared to standard care recipients. - Significant reductions in unplanned coronary revascularizations.
- Notable decreases in patient-reported symptoms, including chest pain, breathlessness, and dizziness.
The TELE-ACS clinical trial, conducted at a large tertiary center in
Erez Nachtomy, CEO of SHL Telemedicine, commented on the significance of these results, stating: "The results of this pivotal study alongside their presentation at ACC 24, and publication in JACC, serve as a resounding vote of confidence in SHL and our SmartHeart® platform as a transformative technology for cardiac care and telemedicine as a whole. These groundbreaking clinical results are an endorsement that propels us forward in our mission to redefine patient care, demonstrating the tangible benefits and efficiency of our telemedicine solutions on a global scale."
For further Information:
- ACC24 Presentation Details: https://www.acc.org/Latest-in-Cardiology/Articles/2024/04/02/17/02/sat-415pm-tele-acs-acc-2024
- JACC Abstract Publication: https://www.jacc.org/doi/10.1016/j.jacc.2024.03.398
About SHL Telemedicine
SHL Telemedicine is engaged in developing and marketing personal telemedicine systems and the provision of medical call center services, with a focus on cardiovascular and related diseases, to end users and to the healthcare community. SHL Telemedicine offers its services and personal telemedicine devices to subscribers utilizing telephonic and Internet communication technology. SHL is listed on the SIX Swiss Exchange (SHLTN, ISIN: IL0010855885, Security No.: 1128957) and on the Nasdaq Stock Exchange (SHLT, ISIN: US78423T2006, CUSIP: 78423T200).
For more information, please visit our website at www.shl-telemedicine.com.
Forward-Looking Statements
Some of the information contained in this press release contains forward-looking statements. Readers are cautioned that any such forward-looking statements are not guarantees of future performance and involve risks and uncertainties, and that actual results may differ materially from those in the forward-looking statements as a result of various factors. SHL Telemedicine undertakes no obligation to publicly update or revise any forward-looking statements.
View source version on businesswire.com: https://www.businesswire.com/news/home/20240410735175/en/
Fabienne Farner, IRF, Phone : +41 43 244 81 42, farner@irf-reputation.ch
Source: SHL Telemedicine Ltd.







