Rezolute Reports Validation of the Potential Use of RZ358 for Treatment of Non-Islet Cell Tumor Hypoglycemia (NICTH)
- Positive results from a preclinical pharmacology study validate the potential of Rezolute's lead compound, RZ358, in treating hypoglycemia in non-islet cell tumors (NICTs).
- RZ358 shows promise in addressing hyperinsulinism caused by various tumor types, expanding the potential patient population for the drug.
- Dr. Brian Roberts highlights the broad utility of RZ358 in treating hypoglycemia resulting from any form of hyperinsulinism, including expanded tumor indications.
- Rezolute plans to initiate a single registrational study with the FDA for patients with hypoglycemia due to tumor hyperinsulinism, potentially including both ICTH and NICTH patients.
- The inclusion of NICTH patients in the potential addressable market for RZ358 would more than double the population, showcasing significant growth opportunities for the company.
- The company is conducting a Phase 3 clinical trial in congenital HI, further demonstrating the versatility and potential therapeutic benefits of RZ358.
- None.
Insights
The recent preclinical pharmacology study conducted by Rezolute, Inc. suggests a significant expansion in the therapeutic scope of RZ358, a compound initially aimed at treating hypoglycemia due to insulinomas. The data indicating RZ358's efficacy against non-islet cell tumor hypoglycemia (NICTH) could have profound implications for the treatment landscape of tumor-induced hypoglycemia. The study's success in demonstrating the compound's ability to blunt IGF-2 mediated insulin-receptor signaling is particularly noteworthy, as it suggests a ligand-agnostic mechanism that could address a wider spectrum of hyperinsulinism causes.
In terms of clinical development, the move towards a single registrational study, as discussed with the FDA, signifies a streamlined path towards potential approval and market entry. The ability to treat both ICTH and NICTH effectively would indeed more than double the addressable patient population, potentially enhancing the commercial viability of RZ358. It's also important to highlight that NICTH represents an unmet medical need, often associated with limited treatment options, which further emphasizes the potential market impact of this compound.
Hypoglycemia in the context of tumor hyperinsulinism presents a complex therapeutic challenge. The findings that RZ358 could be effective in treating hypoglycemia resulting from various tumor types, beyond just insulinomas, is a significant clinical advancement. The ability to manage severe and uncontrolled hypoglycemia in cancer patients is critical, as it can disrupt treatment regimens and significantly impact patient quality of life. The broad utility of RZ358, as indicated by the ligand-agnostic mechanism, could offer a new, valuable tool in the oncologist's arsenal, especially considering the limited treatment options currently available for managing hypoglycemia in these patients.
Moreover, the ongoing Phase 3 clinical trial of RZ358 in congenital HI, a rare pediatric condition, underlines the compound's potential in addressing rare and serious metabolic diseases. If successful, RZ358 could represent a paradigm shift in how clinicians approach the management of hypoglycemia in both oncological and congenital settings, providing a unified treatment strategy for conditions characterized by over-activation of the insulin receptor.
The expansion of RZ358's utility to include NICTH is a strategic move that could significantly enhance Rezolute's market positioning. With the potential to address a larger patient population, the company could see an increase in the addressable market size, which is a key factor in the valuation of biopharmaceutical firms. The anticipation of a single registrational study following successful FDA interactions suggests a favorable regulatory trajectory, which is an important consideration for investors.
Investors should also consider the rarity of congenital HI, which qualifies RZ358 for orphan drug designation, potentially offering benefits such as market exclusivity upon approval. The strategic implications of these developments are considerable, as they not only promise to bring a new treatment to market but also position Rezolute at the forefront of addressing a spectrum of hyperinsulinism-related conditions.
Potential to more than double the addressable patient population living with hypoglycemia resulting from insulin receptor over-activation (tumor hyperinsulinism)
REDWOOD CITY, Calif., March 06, 2024 (GLOBE NEWSWIRE) -- Rezolute, Inc. (Nasdaq: RZLT) (“Rezolute” or the “Company”), a clinical-stage biopharmaceutical company committed to developing novel, transformative therapies for serious metabolic and rare diseases, today announced results from a preclinical pharmacology study that validate the potential for its lead clinical compound, RZ358, to treat individuals with non-islet cell tumors (NICTs) that have uncontrolled hypoglycemia.
Tumor hyperinsulinism (HI) may be caused by a variety of different tumor types, resulting in islet cell tumor hypoglycemia (ICTH) and NICTH. The Company previously reported on the successful use of RZ358 under its Expanded Access Program (EAP) to treat patients with insulin-producing pancreatic islet cell tumors (ICTs), or insulinomas, causing severe and uncontrolled hypoglycemia. The therapeutic potential of RZ358 in this setting was anticipated given that ICTH is mediated by insulin and that RZ358 is known to work at the insulin receptor to decrease excess insulin binding and activity. However, it was unknown if RZ358 would have utility in NICTH where hyperinsulinism is mediated by hormones such as insulin-like growth factor-2 (IGF-2) or its variants, which likewise cause hypoglycemia by binding to and activating the insulin receptor. To test this, the Company recently completed in vitro pharmacology studies to evaluate the impact of clinically relevant concentrations of RZ358 on insulin receptor activation by IGF-2, compared to insulin. This was tested at the relative concentrations of each ligand that activate the insulin receptor and are physiologically relevant in tumor HI caused by ICTH and NICTH, respectively. These experiments successfully demonstrated the ability of RZ358 to similarly blunt both IGF-2 and insulin-mediated insulin-receptor signaling, at levels of these ligands that are disease-relevant in humans.
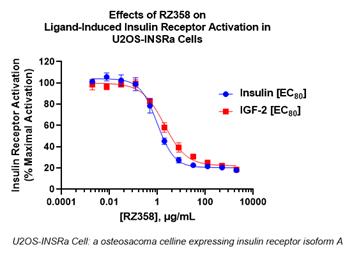
“These data demonstrate proof of the ligand-agnostic mechanism of action of RZ358 and therefore validate its broad utility in treating hypoglycemia resulting from any form of hyperinsulinism, including expanded tumor indications,” said Dr. Brian Roberts, Chief Medical Officer of Rezolute. “Coupled with known outcomes from our clinical trials in congenital HI and the positive outcomes seen with ICTH in our expanded access program, we are excited by the potential for RZ358 to provide dramatic therapeutic benefit to cancer patients who often have limited treatment options for managing serious and uncontrolled hypoglycemia, which can accompany their cancer and disrupt treatment plans.”
The Company recently reported on its successful interaction with the U.S. Food and Drug Administration (FDA) in January 2024 regarding the potential to initiate a single registrational study in patients with hypoglycemia due to tumor HI. The Company will continue to evaluate the feasibility of a development program in this indication, with the possibility of including both ICTH and NICTH patients. The inclusion of NICTH patients in a potential addressable market for RZ358 in tumor HI would more than double the population. The Company is also currently evaluating RZ358 in a Phase 3 clinical trial in congenital HI, which is a rare pediatric condition where, similar to ICTH, children overproduce insulin creating a dangerous hypoglycemic state.
About Tumor Hyperinsulinism (HI)
Tumor HI may be the result of two distinct types of tumors: islet cell tumors (ICTs) and non-islet cell tumors (NICTs), both of which lead to hypoglycemia due to excessive activation of the insulin receptor. Insulinomas are the most common type of functional ICT and cause hypoglycemia because of over production of insulin. NICTs can cause hypoglycemia by producing and secreting insulin-like paraneoplastic substances such as IGF-2 or related variants that bind to and activate the insulin receptor. This form of hypoglycemia can occur in more than 15 different tumor types, 60 percent of which are malignant, including hepatocellular carcinoma. The total addressable market for the combined indications causing tumor HI is estimated to be approximately 4,500 patients in the U.S. alone, including approximately 1,500 with ICTH and 3,000 with NICTH. The unique mechanism of action of RZ358 to attenuate excess insulin receptor activation mediated by insulin and related substances makes the therapy a potential universal treatment for hypoglycemia resulting from any form of hyperinsulinism.
About RZ358
RZ358 is a fully human monoclonal antibody that works downstream from the pancreas and other sources of insulin or related paraneoplastic substances, and instead binds to a unique allosteric site on insulin receptors in the liver, fat, and muscle. The antibody counteracts excess insulin receptor activation by insulin and other effector substances (such as IGF-2), thereby improving hypoglycemia. Because RZ358 acts downstream from the pancreas at the insulin receptor, it has the potential to be universally effective at treating hypoglycemia due to congenital HI, regardless of the causative genetic defect, as well as acquired forms of HI such as those mediated by insulinomas (ICTs) and other tumor types (NICTs). RZ358 received Orphan Drug Designation in the United States and European Union for the treatment of congenital HI, as well as Orphan Drug Designation and Pediatric Rare Disease Designation in the U.S. In the Phase 2 RIZE study, participants with congenital HI ages two and older nearly universally achieved significant improvements in hypoglycemia across multiple endpoints, including the primary and key secondary endpoints planned for the sunRIZE study. At doses and exposures that are planned for the Phase 3 study, RZ358 was generally safe and well-tolerated, and resulted in median improvements in hypoglycemia exceeding
About Rezolute, Inc.
Rezolute strives to disrupt current treatment paradigms by developing transformative therapies for devastating rare and chronic metabolic diseases. Its novel therapies hold the potential to both significantly improve outcomes and reduce the treatment burden for patients, treating physicians, and the healthcare system. Rezolute is steadfast in its mission to create profound, positive, and lasting impacts on patients’ lives. Patient, clinician, and advocate voices are integrated in the Company’s drug development process. Rezolute places an emphasis on understanding the patient’s lived experiences, enabling the Company to boldly address a range of severe conditions. For more information, visit www.rezolutebio.com.
Forward-Looking Statements
This release, like many written and oral communications presented by Rezolute and our authorized officers, may contain certain forward-looking statements regarding our prospective performance and strategies within the meaning of Section 27A of the Securities Act and Section 21E of the Securities Exchange Act of 1934, as amended. We intend such forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995 and are including this statement for purposes of said safe harbor provisions. Forward-looking statements, which are based on certain assumptions and describe future plans, strategies, and expectations of Rezolute, are generally identified by use of words such as "anticipate," "believe," "estimate," "expect," "intend," "plan," "project," "seek," "strive," "try," or future or conditional verbs such as "could," "may," "should," "will," "would," or similar expressions. These forward-looking statements include, but are not limited to statements regarding the Innovation Passport designation, the RZ358 Expanded Access Program, the ability of RZ358 to become an effective treatment for congenital hyperinsulinism, the effectiveness or future effectiveness of RZ358 for the treatment of congenital hyperinsulinism, and statements regarding clinical trial timelines for RZ358. Our ability to predict results or the actual effects of our plans or strategies is inherently uncertain. Accordingly, actual results may differ materially from anticipated results. Readers are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date of this release. Except as required by applicable law or regulation, Rezolute undertakes no obligation to update these forward-looking statements to reflect events or circumstances that occur after the date on which such statements were made. Important factors that may cause such a difference include any other factors discussed in our filings with the SEC, including the Risk Factors contained in the Rezolute’s Annual Report on Form 10-K and Quarterly Reports on Form 10-Q, which are available at the SEC’s website at www.sec.gov. You are urged to consider these factors carefully in evaluating the forward-looking statements in this release and are cautioned not to place undue reliance on such forward-looking statements, which are qualified in their entirety by this cautionary statement.
Investors & Media:
Christen Baglaneas
Rezolute, Inc.
cbaglaneas@rezolutebio.com
(508)272-6717
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/165f8dbc-1fce-47b8-ac3f-0cf6fb4170cb








