Preliminary Phase 1b/2 Data for REC-4881 in Familial Adenomatous Polyposis (FAP) Demonstrates Reduced Polyp Burden
- Significant 43% median reduction in polyp burden at Week 13, surpassing other investigational agents (20-30% in 6 months)
- Strong response rate with 83% of patients showing polyp reduction between 31-82%
- 50% of patients achieved ≥1-point improvement in Spigelman stage
- Generally acceptable safety profile with majority of adverse events being Grade 1-2
- Fast Track and Orphan Drug designations from both FDA and European Commission
- One patient (17%) showed substantial polyp burden increase of 595% from baseline
- 16% of patients experienced Grade 3 adverse events
- 3 out of 19 patients discontinued treatment
- One patient progressed from Stage II to Stage IV in Spigelman staging
Insights
Recursion's REC-4881 shows promising 43% median polyp reduction in FAP patients at 13 weeks, though extreme response variability raises questions.
The preliminary data from Recursion's TUPELO trial provides compelling early evidence for REC-4881 in Familial Adenomatous Polyposis (FAP), a disease with no current FDA-approved treatments despite its near 100% lifetime risk of colorectal cancer if left untreated.
The 43% median reduction in polyp burden at just 13 weeks is particularly noteworthy when compared to historical benchmarks of 20-30% reduction at 6 months with other investigational agents. However, the heterogeneity in patient responses is striking - while 5 of 6 patients (83%) showed meaningful reductions between 31-82%, one patient experienced a concerning 595% increase in polyp burden.
The 50% rate of improvement in Spigelman staging (measuring upper GI disease severity) further supports potential clinical benefit, though again with mixed results as one patient progressed from Stage II to IV.
From a safety perspective, the profile appears consistent with MEK inhibitor class effects. The predominantly Grade 1-2 adverse events, with Grade 3 events in 16% of patients and no Grade 4+ events, suggests general tolerability. The presence of decreased left ventricular ejection fraction (LVEF) as a common event warrants cardiac monitoring in future studies.
For the estimated 50,000 FAP patients across the US and EU5, these preliminary results offer hope, but the extremely small sample size (n=6) and significant response variability necessitate cautious interpretation pending more robust data from the ongoing trial.
Recursion's REC-4881 shows promising early efficacy for FAP, exceeding benchmarks with 43% polyp reduction, but small sample size limits conclusions.
Recursion's preliminary TUPELO trial results represent a potential breakthrough for a rare disease with significant unmet need. The 43% median reduction in polyp burden after just 13 weeks of treatment with REC-4881 appears superior to historical benchmarks (20-30% at 6 months) for other investigational agents in FAP.
The regulatory advantages of Fast Track and Orphan Drug designations from both FDA and European Commission could accelerate development timelines and provide market exclusivity if eventually approved. This positions REC-4881 favorably in the treatment landscape for this orphan indication affecting approximately 50,000 patients across major markets.
However, investors should carefully weigh the extremely small efficacy dataset (n=6 patients) and the concerning outlier with dramatic disease worsening (595% increase in polyp burden). This heterogeneity introduces uncertainty about consistent benefit across the broader patient population.
From a safety perspective, the profile appears manageable with predominantly Grade 1-2 events. The 16% rate of Grade 3 events and absence of Grade 4+ toxicities suggest a tolerable profile, supported by the relatively low discontinuation rate (3 of 19 patients).
What's particularly notable is how Recursion identified this approach using their AI-powered Recursion OS platform by analyzing cellular models of APC gene loss. This technology-driven discovery model demonstrates potential value beyond this specific indication and validates their overall platform approach.
While these data likely justify continued development, they remain too preliminary to significantly de-risk the program, with much depending on whether the efficacy signals persist in larger patient cohorts with longer follow-up.
- In the Phase 2 open-label study, REC-4881 (4 mg QD) led to a median
43% reduction (n=6 patients) in polyp burden at the Week 13 assessment at time of data cutoff. - Five of six patients (
83% ) experienced reductions in polyp burden ranging from31% to82% , however, one patient showed a substantial increase from baseline. - At Week 13,
50% of patients (3 out of 6) achieved ≥1-point improvement in Spigelman stage, a measure of upper GI disease severity. - The early safety profile of REC-4881 was generally consistent with that of prior MEK1/2 inhibitors; among 19 patients across Phase 1b and 2, most treatment-related adverse events were Grade 1 or 2, with Grade 3 events in
16% of patients and no Grade ≥4 TRAEs reported to date.
SALT LAKE CITY, May 04, 2025 (GLOBE NEWSWIRE) -- Recursion (Nasdaq: RXRX), a clinical-stage TechBio company decoding biology to radically improve lives, today announced preliminary safety and efficacy results from its ongoing Phase 1b/2 TUPELO trial of REC-4881, an investigational, allosteric MEK1/2 inhibitor in development for Familial Adenomatous Polyposis (FAP). These data were presented in a late-breaking oral presentation at Digestive Disease Week (DDW) 2025 in San Diego, California.
FAP is a rare, inherited disorder caused by mutations in the APC gene, leading to the growth of hundreds to thousands of gastrointestinal polyps and a near
As of the March 17, 2025 data cutoff in the open-label Phase 2 portion of the TUPELO trial, treatment with REC-4881 (4 mg QD) led to a preliminary median
Across the combined Phase 1b and ongoing Phase 2 cohorts (n=19 safety-evaluable patients), REC-4881 demonstrated an early safety profile generally consistent with that of prior MEK1/2 inhibitors. Seventy-nine percent (15 of 19) of patients experienced at least one treatment-related adverse event (TRAE), the majority of which were Grade 1 or 2 in severity. Grade 3 TRAEs occurred in
"For patients with FAP, who face increased risk of colorectal and small bowel cancer and a lifetime of invasive interventions, the preliminary polyp burden reduction at a median of >
"For patients with FAP, who currently lack FDA-approved treatment options, Recursion's AI-powered Recursion OS platform identified a promising approach through MEK 1/2 inhibition," said Najat Khan, PhD, Chief R&D Officer and Chief Commercial Officer at Recursion. "By analyzing cellular models of APC gene loss, we uncovered a potential first-in-disease treatment and are excited to share our preliminary findings."
About the TUPELO Trial Design (REC-4881)
The Phase 1b/2 TUPELO trial is evaluating the safety, tolerability, pharmacokinetics (PK), and preliminary efficacy of REC-4881 monotherapy in patients with Familial Adenomatous Polyposis (FAP).
The Phase 1b portion was a randomized, double-blind, placebo-controlled safety run-in designed to assess the safety, tolerability, and PK of REC-4881 at 4 mg QD for 14 days in FAP patients aged 18 years and older. A total of five patients received REC-4881, and two received placebo. Following the safety review, the eligibility criteria were amended to enroll only patients aged ≥55 years in Phase 2, in an effort to reduce treatment-related adverse events (TRAEs) consistent with MEK1/2 inhibition.
In the ongoing Phase 2 portion, participants must have a confirmed germline APC mutation and be ≥55 years old. Efficacy is assessed via upper and lower endoscopy at baseline, Week 13 (W13, on-treatment), and Week 25 (W25, off-treatment). The primary efficacy endpoint is percent change from baseline in polyp burden.
The Efficacy Evaluable Population includes patients who had measurable disease at baseline, received ≥
Efficacy
In the interim Phase 2 analysis, REC-4881 (4 mg QD) demonstrated a preliminary median
At Week 13, three of six patients achieved a ≥1-point reduction in Spigelman stage, a validated measure of upper gastrointestinal disease severity; one patient had no change, one progressed from Stage II to IV, and one had no baseline stage but was classified as Stage II at Week 13.
At the time of data cutoff, three additional patients had been enrolled in the 8 mg QD dose cohort. All three were found to have no measurable disease (zero polyp burden) at baseline endoscopy and were therefore not considered efficacy evaluable.
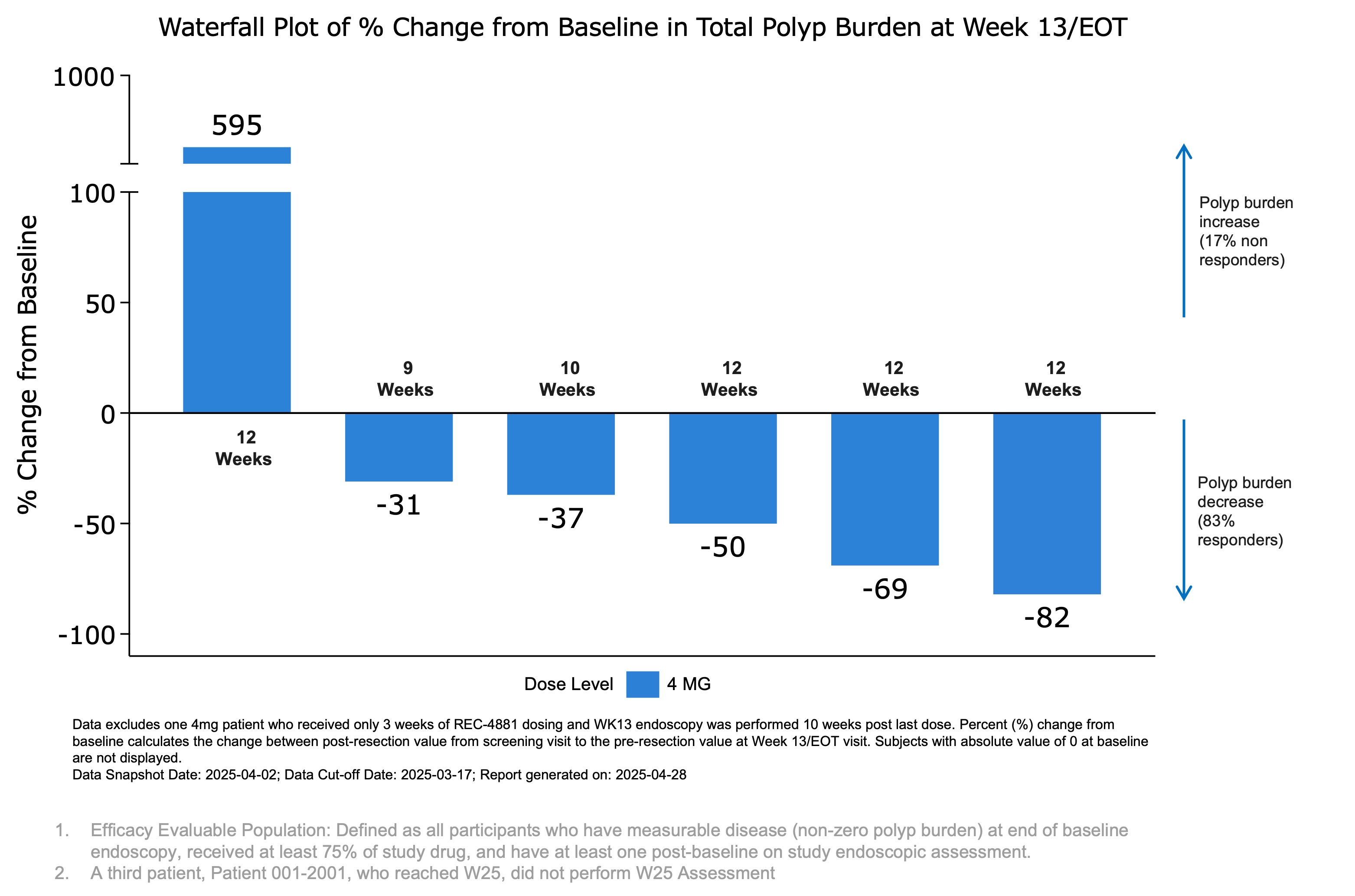
Figure 1: Waterfall plot showing percent change from baseline in total polyp burden at Week 13 (end of treatment) for efficacy-evaluable patients receiving REC-4881 (4 mg QD).
Safety
In the preliminary Phase 1b safety and Phase 2 dose-finding/dose-expansion cohorts of the TUPELO trial, the majority of treatment-related adverse events (TRAEs) were Grade 1 or 2, with Grade 3 TRAEs observed in
Grade 3 TRAEs included rash, increased C-reactive protein (CRP), and decreased LVEF—each occurring in one patient (
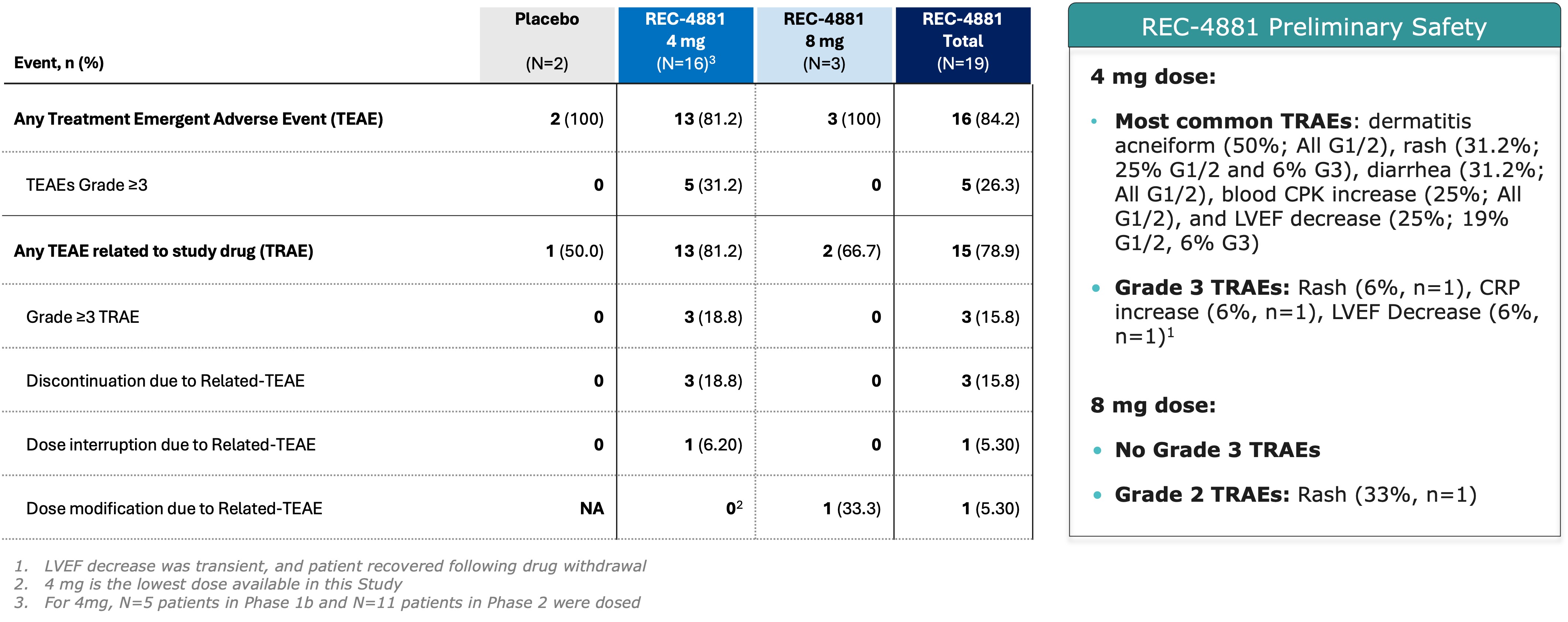
Figure 2. Summary of Adverse Events Across Phase 1b and Phase 2 of the TUPELO Trial
Next Steps
Patient enrollment in the TUPELO trial is ongoing. Recursion plans to conduct additional efficacy and safety analyses anticipated in the second half of 2025.
About REC-4881
REC-4881 is an orally bioavailable, non-ATP-competitive allosteric small molecule MEK 1/2 inhibitor. The platform analyzed cellular models of APC gene loss—the root cause of FAP—to identify compounds that suppress the disease-inducing effects of APC mutations.
About Recursion
Recursion (NASDAQ: RXRX) is a clinical stage TechBio company leading the space by decoding biology to radically improve lives. Enabling its mission is the Recursion OS, a platform built across diverse technologies that continuously generate one of the world’s largest proprietary biological and chemical datasets. Recursion leverages sophisticated machine-learning algorithms to distill from its dataset a collection of trillions of searchable relationships across biology and chemistry unconstrained by human bias. By commanding massive experimental scale — up to millions of wet lab experiments weekly — and massive computational scale — owning and operating one of the most powerful supercomputers in the world, Recursion is uniting technology, biology and chemistry to advance the future of medicine. Recursion is headquartered in Salt Lake City, where it is a founding member of BioHive, the Utah life sciences industry collective. Recursion also has offices in Toronto, Montréal, New York, London, Oxford area, and the San Francisco Bay area. Learn more at www.Recursion.com, or connect on X (formerly Twitter) and LinkedIn.
Forward-Looking Statements
This document contains information that includes or is based upon "forward-looking statements" within the meaning of the Securities Litigation Reform Act of 1995, including, without limitation, those regarding the potential efficacy of REC-4881, including the potential to be a first-in-disease treatment; the timing of and outcomes of the TUPELO clinical trial; the effects of our platform on trial outcomes; the potential size of the market opportunity for our drug candidates; Recursion’s leadership of the TechBio space; the Recursion OS and other technologies; and all other statements that are not historical facts. Forward-looking statements may or may not include identifying words such as “plan,” “will,” “expect,” “anticipate,” “intend,” “believe,” “potential,” “continue,” and similar terms. These statements are subject to known or unknown risks and uncertainties that could cause actual results to differ materially from those expressed or implied in such statements, including but not limited to: challenges inherent in pharmaceutical research and development, including the timing and results of preclinical and clinical programs, where the risk of failure is high and failure can occur at any stage prior to or after regulatory approval due to lack of sufficient efficacy, safety considerations, or other factors; our ability to leverage and enhance our drug discovery platform; our ability to obtain financing for development activities and other corporate purposes; the success of our collaboration activities; our ability to obtain regulatory approval of, and ultimately commercialize, drug candidates; our ability to obtain, maintain, and enforce intellectual property protections; cyberattacks or other disruptions to our technology systems; our ability to attract, motivate, and retain key employees and manage our growth; inflation and other macroeconomic issues; and other risks and uncertainties such as those described under the heading “Risk Factors” in our filings with the U.S. Securities and Exchange Commission, including our Annual Report on Form 10-K for the Fiscal Year Ended December 31, 2024. All forward-looking statements are based on management’s current estimates, projections, and assumptions, and Recursion undertakes no obligation to correct or update any such statements, whether as a result of new information, future developments, or otherwise, except to the extent required by applicable law.
Media Contact
Media@Recursion.com
Investor Contact
Investor@Recursion.com
Photos accompanying this announcement are available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/d025ef61-3451-47e1-9dc6-8fe910b4ef6d
https://www.globenewswire.com/NewsRoom/AttachmentNg/b64bdf18-73b8-4f07-b38d-a96478162b1b
1 Steinberg et al 2000 NEJM and Burke et al 2024 Gastroenterology








