Revance Announces the Launch of OPUL™, the First-of-its-Kind Relational Commerce Platform for Aesthetic Practices
-OPUL is an end-to-end, cloud-based payment platform designed to cultivate long-term customer relationships and optimize business operations for aesthetic practices-
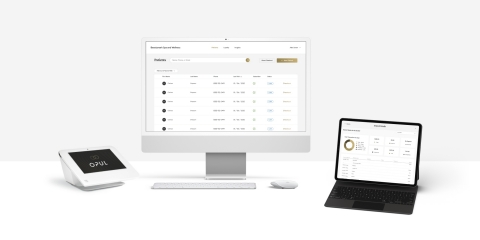
Opul - First-of-its-Kind Relational Commerce Platform for Aesthetic Practices (Photo: Business Wire)
Today’s aesthetic market is flooded with discounts and coupons, driving consumers to price shop. The result for practices is a decrease in consumer loyalty and retention. As the latest launch from the Revance Aesthetics portfolio, OPUL™ is the solution that replaces one-and-done transactions with a more valuable and profitable relationship model. Early release features include:
- Practice Reporting and Analytics: Comprehensive reporting to help aesthetic practice owners and managers understand the health of their business with transaction and sales data across all products and services – not limited to one brand.
- Customizable Checkout: Customizable check out options to elevate consumer experiences, including a comprehensive catalog concierge with access to over 6,000 aesthetic products and services.
- Seamless and Smart Payments: OPUL operates as a registered payment facilitator (PayFac), enabling OPUL to offer low and transparent processing fees, which helps to increase transaction value for the practice, and provides trackable insights of purchasing history to help encourage reoccurring visits and consumer loyalty.
“OPUL™ was built to address the important needs of aesthetic practices today – optimizing patient experiences and business outcomes through strong customer loyalty and relationships. With almost 40,000 and growing aesthetic practices across the
We will continue to offer the HintMD fintech platform, the fintech platform provided by
For practices interested in learning more about OPUL™ or to request a demonstration, please visit OPUL.com.
About Revance
Revance is a biotechnology company focused on innovative aesthetic and therapeutic offerings, including its next-generation neuromodulator product, DaxibotulinumtoxinA for Injection. DaxibotulinumtoxinA for Injection combines a proprietary stabilizing peptide excipient with a highly purified botulinum toxin that does not contain human or animal-based components. Revance has successfully completed a Phase 3 program for DaxibotulinumtoxinA for Injection in glabellar (frown) lines and is pursuing
“Revance Therapeutics” and the Revance logo are registered trademarks of
Resilient Hyaluronic Acid® and RHA® are trademarks of
BOTOX® is a registered trademark of
Forward Looking Statements
Any statements in this press release that are not statements of historical fact, including statements related to the potential benefits to practices and patients of our drug product candidates and our technologies, including DaxibotulinumtoxinA for Injection, if approved, the RHA® Collection of dermal fillers and OPUL™; the needs of aesthetic practices; the rate and degree of commercial acceptance, opportunity and growth potential of OPUL™; the aesthetics industry and the size and growth of the aesthetics market; the growth opportunities available to the company; our ability to set a new standard in healthcare; differentiation of our products and services in comparison to our competitors; development of a biosimilar to BOTOX®; and statements about our business strategy, timeline and other goals, plans and prospects, constitute forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. You should not rely upon forward-looking statements as predictions of future events. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance, events, circumstances or achievements reflected in the forward-looking statements will ever be achieved or occur.
Forward-looking statements are subject to risks and uncertainties that could cause actual results to differ materially from our expectations. These risks and uncertainties relate, but are not limited to: the results, timing, costs, and completion of our research and development activities and regulatory approvals, including the continuing delay in the FDA’s approval of the BLA for DaxibotulinumtoxinA for Injection for the treatment of glabellar lines, including as a result of observations made by the FDA during the site inspection or other reasons; the impact of the COVID-19 pandemic on our manufacturing operations, supply chain, end user demand for our products, commercialization efforts, business operations, clinical trials and other aspects of our business and on the market; our ability to manufacture supplies for our product candidates and to acquire supplies of the RHA® Collection of dermal fillers; the uncertain clinical development process; the risk that clinical trials may not have an effective design or generate positive results or that positive results would assure regulatory approval or commercial success; the applicability of clinical study results to actual outcomes; the rate and degree of economic benefit, the safety, efficacy, commercial acceptance and the market, competition, size and growth potential of OPUL™, the RHA® Collection of dermal fillers and our dug product candidates, if approved; our ability to continue to successfully commercialize the RHA® Collection of dermal fillers and OPUL™ and our ability to successfully commercialize DaxibotulinumtoxinA for Injection, if approved, and the timing and cost of commercialization activities; our ability to expand sales and marketing capabilities; the status of commercial collaborations; our ability to obtain funding for our operations; the cost and our ability to defend ourselves in product liability, intellectual property and other lawsuits; our ability to continue obtaining and maintaining intellectual property protection for our drug product candidates; our financial performance, including future revenue, expenses and capital requirements; and other risks. Detailed information regarding factors that may cause actual results to differ materially from the results expressed or implied by statements in this press release may be found in our periodic filings with the
View source version on businesswire.com: https://www.businesswire.com/news/home/20211011005200/en/
Media
sfahy@revance.com
Investors
Jessica.serra@revance.com
or
laurence@gilmartinir.com
Source:







