Relmada Therapeutics Announces Efficacy and Safety Results from the Phase 3 Long-Term Study of REL-1017 in Major Depressive Disorder
- Patients newly treated with REL-1017 for up to one year experienced rapid, clinically meaningful, and sustained improvements in depressive symptoms and associated functional impairment
- Long-term dosing with REL-1017 was well-tolerated, with low rates of adverse events and discontinuations due to adverse events, and no new safety signals were detected
Patients treated daily with REL-1017 for up to one year experienced rapid, clinically meaningful, and sustained improvements in depressive symptoms and associated functional impairment. REL-1017 was well-tolerated with long-term dosing, showing low rates of adverse events and discontinuations due to adverse events. No new safety signals were detected.
"These efficacy and safety results represent real-world potential outcomes for MDD patients when treated with REL-1017," said Cedric O'Gorman, MD, Chief Medical Officer of Relmada. "The rapid and sustained therapeutic effects achieved with REL-1017 suggest the significant therapeutic potential of this promising late-stage product candidate as a mechanistically novel and differentiated treatment for MDD. The early magnitude and trajectory of clinical improvement remain consistent across all trials conducted to date. The long-term sustained clinical improvement, coupled with an extremely well-tolerated profile, adds to our enthusiasm for this agent as a potential therapeutic option for patients and prescribers."
Study REL-1017-310 was a long-term, open-label, non-comparative, registrational Phase 3 trial designed to evaluate the efficacy and safety of REL-1017 administered once-daily in patients with MDD for up to one year. In total, 627 patients were enrolled, comprising 423 patients who rolled over (rollover patients/subjects) from placebo-controlled trials with REL-1017 (Studies 301, 302 and 303), and 204 de novo patients who had not previously participated in trials with REL-1017. The trial was concluded when at least 300 patients had been treated for six months and approximately 100 patients had been treated for 12 months. At the time of study conclusion, 418 patients had reached at least six months of treatment, and 118 patients had reached at least 12 months of treatment.
Efficacy results are presented below for de novo patients only.
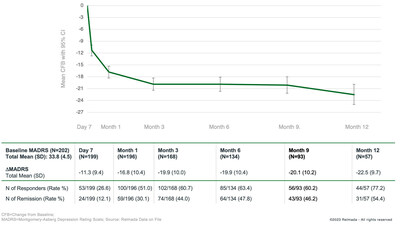
Rapid and Sustained Substantial Improvement in MADRS Total Score over Time
In de novo patients, the mean MADRS total score was 33.8 at baseline. Treatment with REL-1017 in these patients resulted in mean improvements from baseline in the MADRS total score of 11.3 points at Day 7, 16.8 points at Month 1, 19.9 points at Months 3 and 6, and 22.5 points at Month 12.
High Rates of Clinical Response, Both Rapid and Sustained
When treated with REL-1017,
Meaningful Rates of Clinical Remission
The virtual absence of depressive symptoms (clinical remission) was achieved by
CGI-I Scale Also Showed Clinical Improvement
As assessed by the Clinical Global Impression of Improvement (CGI-I) Scale, de novo treated patients showed meaningful improvements consistent with MADRS efficacy improvements.
Significant Reduction in Functional Impairment associated with MDD
Functional impairment across all three separate domains of the Sheehan Disability Scale was improved with REL-1017 treatment by an average of approximately
Symptoms of Anxiety in MDD Reduced with REL-1017 Treatment
The mean baseline score on the Hamilton Anxiety Rating Scale (HAM-A), which measures symptoms of anxiety, was 20.6 points, reflecting moderate anxiety. De novo patients treated with REL-1017 saw a continual decline in anxiety symptoms over time as measured by the HAM-A. Score improvements were 7.1 points at Day 7, 9.6 points at Month 1, 11.1 points at Month 3, 11.5 points at Month 6, and 13.5 points at Month 12.
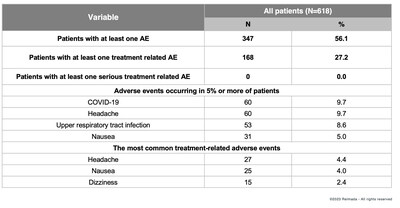
Long-Term Safety and Tolerability Results
REL-1017 was safe and well-tolerated with a profile consistent with that observed in short-term controlled trials, with no new safety signals detected. Safety results presented for Study REL-1017-310 are for all patients (de novo and rollover) in the trial.
Discontinuations due to adverse events occurred in approximately
Importantly, there was no significant safety signal for weight gain, sexual dysfunction, cardiovascular issues, dissociative effects, withdrawal phenomena or abuse liability.
About REL-1017
REL-1017, a new chemical entity (NCE) and novel NMDA receptor (NMDAR) channel blocker that preferentially targets hyperactive channels while maintaining physiological glutamatergic neurotransmission, is currently in late-stage development for the adjunctive treatment of major depressive disorder (MDD). The ongoing Clinical Research Program is designed to evaluate the potential for REL-1017 as a rapid-acting, oral, once-daily antidepressant treatment. In addition to the long-term, open-label study of REL-1017, the Phase 3 development program for REL-1017 as an adjunctive treatment for MDD also includes the recently initiated Relight (Study 304) Phase 3, randomized, double-blind, placebo-controlled trial and the ongoing Reliance II (Study 302) trial. Relight and Reliance II have the same key study design parameters.
About Relmada Therapeutics, Inc.
Relmada Therapeutics is a late-stage biotechnology company addressing diseases of the central nervous system (CNS), with a focus on major depressive disorder (MDD). Relmada's experienced and dedicated team is committed to making a difference in the lives of patients and their families. Relmada's lead program, REL-1017, is a new chemical entity (NCE) and novel NMDA receptor (NMDAR) channel blocker that preferentially targets hyperactive channels while maintaining physiological glutamatergic neurotransmission. REL-1017 is in late-stage development as an adjunctive treatment for MDD in adults. Learn more at www.relmada.com.
Forward-Looking Statements
The Private Securities Litigation Reform Act of 1995 provides a safe harbor for forward-looking statements made by us or on our behalf. This press release contains statements which constitute "forward-looking statements" within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Any statement that is not historical in nature is a forward-looking statement and may be identified by the use of words and phrases such as "expects," "anticipates," "believes," "will," "will likely result," "will continue," "plans to," "potential," "promising," and similar expressions. These statements are based on management's current expectations and beliefs and are subject to a number of risks, uncertainties and assumptions that could cause actual results to differ materially from those described in the forward-looking statements, including potential failure of clinical trial results to demonstrate statistically and/or clinically significant evidence of efficacy and/or safety, failure of top-line results to accurately reflect the complete results of the trial, failure of the 310 open-label study to accurately reflect the results of the ongoing 302 and 304 blinded, randomized and controlled studies, failure to obtain regulatory approval of REL-1017 for the treatment of major depressive disorder, and the other risk factors described under the heading "Risk Factors" set forth in the Company's reports filed with the SEC from time to time. No forward-looking statement can be guaranteed, and actual results may differ materially from those projected. Relmada undertakes no obligation to publicly update any forward-looking statement, whether as a result of new information, future events, or otherwise. Readers are cautioned that it is not possible to predict or identify all the risks, uncertainties and other factors that may affect future results and that the risks described herein should not be a complete list.
Investor Contact:
Tim McCarthy
LifeSci Advisors
tim@lifesciadvisors.com
Media Inquiries:
Corporate Communications
media@relmada.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/relmada-therapeutics-announces-efficacy-and-safety-results-from-the-phase-3-long-term-study-of-rel-1017-in-major-depressive-disorder-301933333.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/relmada-therapeutics-announces-efficacy-and-safety-results-from-the-phase-3-long-term-study-of-rel-1017-in-major-depressive-disorder-301933333.html
SOURCE Relmada Therapeutics, Inc.