REGENXBIO INITIATES PIVOTAL PHASE OF AFFINITY DUCHENNE® TRIAL OF RGX-202 GENE THERAPY AND REPORTS POSITIVE FUNCTIONAL DATA
REGENXBIO has initiated the pivotal phase of AFFINITY DUCHENNE® trial for RGX-202, a gene therapy for Duchenne muscular dystrophy, with FDA alignment on accelerated approval pathway and expected BLA in 2026. The trial reports positive functional data across both dose levels, with improvements in North Star Ambulatory Assessment scores and timed function tests. New biomarker data shows consistent robust expression of RGX-202 microdystrophin in muscle tissue, with the highest reported levels among gene therapies. The pivotal trial will evaluate approximately 30 ambulatory patients aged 1 and older, with no serious adverse events reported to date.
REGENXBIO ha avviato la fase cruciale dello studio AFFINITY DUCHENNE® per RGX-202, una terapia genica per la distrofia muscolare di Duchenne, con allineamento della FDA su un percorso di approvazione accelerata e una BLA prevista per il 2026. Lo studio riporta dati funzionali positivi su entrambi i livelli di dose, con miglioramenti nei punteggi della North Star Ambulatory Assessment e nei test di funzione temporizzati. Nuovi dati sui biomarcatori mostrano un'espressione robusta e costante della microdistrofina RGX-202 nei tessuti muscolari, con i livelli riportati più elevati tra le terapie geniche. Lo studio cruciale valuterà circa 30 pazienti ambulatoriali di età pari o superiore a 1 anno, senza eventi avversi grave riportati fino ad oggi.
REGENXBIO ha iniciado la fase pivotal del ensayo AFFINITY DUCHENNE® para RGX-202, una terapia génica para la distrofia muscular de Duchenne, con alineación de la FDA sobre un camino de aprobación acelerada y una BLA esperada para 2026. El ensayo reporta datos funcionales positivos en ambos niveles de dosis, con mejoras en las puntuaciones de la North Star Ambulatory Assessment y en las pruebas de función cronometradas. Los nuevos datos de biomarcadores muestran una expresión robusta y consistente de microdistrofina RGX-202 en el tejido muscular, con los niveles más altos reportados entre las terapias génicas. El ensayo pivotal evaluará aproximadamente a 30 pacientes ambulatorios de 1 año o más, sin eventos adversos graves reportados hasta la fecha.
REGENXBIO가 두센형 근위축증을 위한 유전자 치료제 RGX-202에 대한 AFFINITY DUCHENNE® 시험의 중요한 단계를 시작했습니다. FDA와의 협의를 통해 가속 승인의 경로가 확립되었으며, 2026년까지 BLA가 예상됩니다. 이 시험은 두 가지 용량 수준에 걸쳐 긍정적인 기능적 데이터를 보고하며, North Star Ambulatory Assessment 점수 및 시간을 기준으로 한 기능 테스트에서 개선이 있었습니다. 새로운 바이오마커 데이터는 RGX-202 마이크로디스트로핀의 근육 조직 내 강력하고 일관된 발현을 보여주며, 유전자 치료제 중에서 가장 높은 수준이 보고되었습니다. 이 중요한 시험은 1세 이상의 약 30명의 보행 환자를 평가할 예정이며, 현재까지 심각한 부작용은 보고되지 않았습니다.
REGENXBIO a lancé la phase décisive de l'essai AFFINITY DUCHENNE® pour RGX-202, une thérapie génique pour la dystrophie musculaire de Duchenne, avec un alignement de la FDA sur un chemin d'approbation accéléré et une BLA prévue pour 2026. L'essai rapporte des données fonctionnelles positives sur les deux niveaux de dose, avec des améliorations des scores de l'évaluation ambulatoire North Star et des tests de fonction chronométrés. De nouvelles données sur les biomarqueurs montrent une expression robuste et cohérente de la microdystrophine RGX-202 dans le tissu musculaire, avec les niveaux les plus élevés rapportés parmi les thérapies géniques. L'essai décisif évaluera environ 30 patients ambulatoires âgés d'un an ou plus, sans événements indésirables graves signalés à ce jour.
REGENXBIO hat die entscheidende Phase der AFFINITY DUCHENNE®-Studie für RGX-202, eine Gentherapie für die Duchenne-Muskeldystrophie, eingeleitet, mit einer Abstimmung der FDA über einen beschleunigten Genehmigungsweg und einer erwarteten BLA im Jahr 2026. Die Studie berichtet über positive funktionale Daten in beiden Dosierungsstufen, mit Verbesserungen bei den Ergebnissen der North Star Ambulatory Assessment und bei zeitbasierten Funktionstests. Neue Biomarker-Daten zeigen eine konsistente, robuste Expression von RGX-202-Mikrodystrophin im Muskelgewebe, mit den höchsten berichteten Werten unter den Gentherapien. Die entscheidende Studie wird etwa 30 ambulante Patienten im Alter von 1 Jahr und älter bewerten, wobei bis heute keine schwerwiegenden Nebenwirkungen berichtet wurden.
- None.
- None.
Insights
The initiation of RGX-202's pivotal trial phase represents a significant milestone in Duchenne muscular dystrophy treatment development. The data shows robust efficacy markers with microdystrophin expression levels reaching up to
The safety profile is particularly encouraging, with no serious adverse events or special interest events reported across all dose levels. The FDA alignment on accelerated approval pathway and planned BLA submission in 2026 significantly de-risks the regulatory process. The expansion to treat patients as young as 1 year old provides a unique market position, as REGENXBIO is currently the only company recruiting this age group in the U.S.
This development positions REGENXBIO favorably in the lucrative Duchenne market. The accelerated approval pathway could lead to faster commercialization, potentially generating revenue streams by 2026-2027. The robust efficacy data and unique positioning for younger patients (ages 1-4) provide significant competitive advantages in a market where current treatment options are
The comprehensive data package, including superior microdystrophin expression levels and functional improvements, strengthens the company's potential market position. With a current market cap of
- Alignment achieved with FDA on AFFINITY DUCHENNE® pivotal program and access to accelerated approval; BLA expected in 2026
- Pivotal trial of RGX-202 is enrolling ambulatory patients aged 1 and above with first patient dosed
- Phase I/II data show RGX-202 recipients exceeding external natural history and established benchmarks for clinical outcomes
- Functional improvements seen in all patients treated with dose level 1 and dose level 2 at 12 and 9 months respectively
- New biomarker data confirms consistent robust expression of differentiated RGX-202 microdystrophin in the muscle
- Favorable safety profile observed at both dose levels; no serious adverse events or AEs of special interest
- Webcast to be held at 8:00 a.m. today
"The initiation of our pivotal trial and newly released positive functional data are exciting milestones on our path to rapidly deliver RGX-202, the only next generation gene therapy in pivotal phase, to the Duchenne community," said Curran M. Simpson President and Chief Executive Officer of REGENXBIO. "The totality of our data demonstrates that RGX-202 provides evidence of improving outcomes for boys with Duchenne and altering the trajectory of this devastating disease, with consistent, robust expression of our novel microdystrophin translating into significant clinical benefit. Based on the strength of the Phase I/II data and our positive discussions and alignment with the FDA, we are quickly advancing RGX-202 toward a BLA filing in 2026 using the accelerated approval pathway. We continue to evaluate opportunities to expand the RGX-202 program to benefit Duchenne patients worldwide."
"There remains a critical need for new therapeutic options for patients with Duchenne muscular dystrophy", said Aravindhan Veerapandiyan M.D., Arkansas Children's Hospital. "I am very pleased to see the advancement of the RGX-202 program to the pivotal stage, which offers promise for a broader patient population and am highly encouraged by the functional data presented today demonstrating RGX-202's potential to alter the course of the disease. The safety, functional, and biomarker data shared today reinforce the positive feedback from families, highlighting improvements in patients' daily activities and underscoring the potential benefits of this treatment."
AFFINITY DUCHENNE Data Updates
Functional Data
Today, REGENXBIO announced positive functional results from the first five participants in the Phase I/II portion of the ongoing AFFINITY DUCHENNE trial. Results include 12-month data from three dose level 1 patients aged 4-10 and nine-month data from two dose level 2 (pivotal dose) patients aged 8 and 12.
In all five participants, across both dose levels, RGX-202 demonstrates evidence of positively impacting disease trajectory, with patients demonstrating stable or improved function on the North Star Ambulatory Assessment (NSAA) and timed function tests. Results were measured against external natural history controls matched for age and baseline function.
Pivotal Dose Functional Data
Pivotal dose participants demonstrated improved performance on NSAA and timed function tests at nine months, exceeding external natural history controls. The NSAA mean score at this dose improved by 5.5 points. [Figure 1]
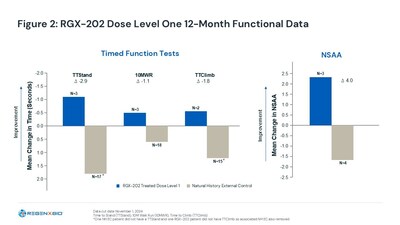
All dose level 1 participants demonstrated improved performance and exceeded external natural history controls at 12 months. [Figure 2]
Additionally, dose level 1 participants' timed task velocity changes exceeded minimal clinically important difference (MCID) benchmarks at 12 months, a measure referenced by the FDA in the approval of the available gene therapy.
Biomarker Data
REGENXBIO also announced new biomarker data that continues to support consistent, high expression and transduction of RGX-202 microdystrophin. RGX-202 was appropriately localized to the sarcolemma, demonstrating the differentiated construct with the CT-Domain is appropriately targeting the muscle.
RGX-202 microdystrophin expression results in ambulatory patients aged 8+ are the highest reported microdystrophin levels across approved or investigational gene therapies.
Mean at 12 Weeks (min,max) | Dose Level 1 1x1014 GC/kg | Dose Level 2 2x1014 GC/kg | ||
Age range (number with data) | 4-7 (2) | 8-11 | 4-7 | 8-11 |
RGX-202 Microdystrophin % normal control (Western Blot) | 60.6 (37.8, 83.4) | 10.4 (n/a) | 77.2 (n/a) | 39.7 |
VCN copies/nucleus (qPCR) | 9.8 (7.4,12.1) | 5.4 (n/a) | 55.4 (n/a) | 17.8 (12.0,30.7) |
Positive Fibers % (Immunofluorescence) | 79.3 (n/a) | 34.6 (n/a) | 71.1 (n/a) | 45.7 (21.3,70.6) |
Safety and Tolerability Data
As of November 1, 2024, RGX-202 was well tolerated with no serious adverse events (SAEs) and no AEs of special interest (AESIs). Common drug-related AEs included nausea, vomiting and fatigue. All resolved and are typically anticipated with gene therapy administration.
RGX-202 Treatment | Dose Level 1 Dose Evaluation (1x1014 GC/kg)
| Dose Level 2 Dose Evaluation / Expansion (2x1014 GC/kg)
| Dose Level 2 Younger Boys (2x1014 GC/kg)
| Total n=11 | |
Age Range (number dosed) | 4-11 (n=3) | 4-11 (n=7) | 1-3 (n=1) | All Ages | |
SAE | 0 | 0 | 0 | 0 | |
AESI | Central or | 0 | 0 | 0 | 0 |
Drug-induced liver | 0 | 0 | 0 | 0 | |
Thrombocytopenia | 0 | 0 | 0 | 0 | |
Myocarditis | 0 | 0 | 0 | 0 | |
Myositis | 0 | 0 | 0 | 0 | |
Pivotal Study
The Phase I/II AFFINITY DUCHENNE trial has been expanded into a multicenter, open-label pivotal Phase I/II/III trial of RGX-202. The pivotal trial is expected to support a Biologics License Application (BLA) submission using the accelerated approval pathway in 2026.
Based on a positive End of Phase 2 meeting with the FDA, the pivotal trial will evaluate the efficacy of RGX-202 at dose level 2 (2×1014 GC/kg) in approximately 30 ambulatory patients aged 1 and older. Patients under 4 years old have no access to gene therapy, and REGENXBIO is the only gene therapy sponsor recruiting patients in this age group in the
To support accelerated approval, the primary endpoint is the proportion of participants whose RGX-202 microdystrophin expression is ≥
Webcast Details
REGENXBIO will host a webcast featuring Dr. Veerapandiyan, and Michael Kelly, PhD, Chief Scientific Officer of CureDuchenne, to discuss today's developments at 8:00 a.m. EST.
The live webcast can be accessed here and in the Investors section of REGENXBIO's website at www.regenxbio.com. An archived replay of the webcast will be available for approximately 30 days following the presentation.
About RGX-202
RGX-202 is a potential best-in-class investigational gene therapy designed for improved function and outcomes in Duchenne. RGX-202 is the only gene therapy approved or in late-stage development for Duchenne with a differentiated microdystrophin construct that encodes key regions of naturally occurring dystrophin, including the C-Terminal (CT) domain. In preclinical studies, the CT domain has been shown to protect the muscle from contraction-induced stress and improve its ability to repair itself.
Additional design features, including codon optimization and reduction of CpG content, may potentially improve gene expression, increase protein translation efficiency and reduce immunogenicity. RGX-202 is designed to support the delivery and targeted expression of genes throughout skeletal and heart muscle using the NAV® AAV8 vector and a well-characterized muscle-specific promoter (Spc5-12). RGX-202 is manufactured using REGENXBIO's proprietary, high-yielding NAVXpress™ suspension-based platform process.
About Duchenne Muscular Dystrophy
Duchenne is a severe, progressive, degenerative muscle disease, affecting 1 in 3,500 to 5,000 boys born each year worldwide. Duchenne is caused by mutations in the Duchenne gene which encodes for dystrophin, a protein involved in muscle cell structure and signaling pathways. Without dystrophin, muscles throughout the body degenerate and become weak, eventually leading to loss of movement and independence, required support for breathing, cardiomyopathy and premature death.
ABOUT REGENXBIO Inc.
REGENXBIO is a leading clinical-stage biotechnology company seeking to improve lives through the curative potential of gene therapy. Since its founding in 2009, REGENXBIO has pioneered the development of AAV Therapeutics, an innovative class of gene therapy medicines. REGENXBIO is advancing a pipeline of AAV Therapeutics for rare and retinal diseases, including RGX-202 for the treatment of Duchenne, RGX-121 for the treatment of MPS II, and ABBV-RGX-314 for the treatment of wet AMD and diabetic retinopathy, being developed in collaboration with AbbVie. Thousands of patients have been treated with REGENXBIO's AAV Therapeutic platform, including Novartis' ZOLGENSMA for children with spinal muscular atrophy. Designed to be one-time treatments, AAV Therapeutics have the potential to change the way healthcare is delivered for millions of people. For more information, please visit www.regenxbio.com.
FORWARD-LOOKING STATEMENTS
This press release includes "forward-looking statements," within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. These statements express a belief, expectation or intention and are generally accompanied by words that convey projected future events or outcomes such as "believe," "may," "will," "estimate," "continue," "anticipate," "assume," "design," "intend," "expect," "could," "plan," "potential," "predict," "seek," "should," "would" or by variations of such words or by similar expressions. The forward-looking statements include statements relating to, among other things, REGENXBIO's future operations, clinical trials, and regulatory plans. REGENXBIO has based these forward-looking statements on its current expectations and assumptions and analyses made by REGENXBIO in light of its experience and its perception of historical trends, current conditions and expected future developments, as well as other factors REGENXBIO believes are appropriate under the circumstances. However, whether actual results and developments will conform with REGENXBIO's expectations and predictions is subject to a number of risks and uncertainties, including the timing of enrollment, commencement and completion and the success of clinical trials conducted by REGENXBIO, its licensees and its partners, the timing of commencement and completion and the success of preclinical studies conducted by REGENXBIO and its development partners, the timely development and launch of new products, the ability to obtain and maintain regulatory approval of product candidates, the ability to obtain and maintain intellectual property protection for product candidates and technology, trends and challenges in the business and markets in which REGENXBIO operates, the size and growth of potential markets for product candidates and the ability to serve those markets, the rate and degree of acceptance of product candidates, and other factors, many of which are beyond the control of REGENXBIO. Refer to the "Risk Factors" and "Management's Discussion and Analysis of Financial Condition and Results of Operations" sections of REGENXBIO's Annual Report on Form 10-K for the year ended December 31, 2023, and comparable "risk factors" sections of REGENXBIO's Quarterly Reports on Form 10-Q and other filings, which have been filed with the
Zolgensma® is a registered trademark of Novartis AG. All other trademarks referenced herein are registered trademarks of REGENXBIO.
Contacts:
Dana Cormack
Corporate Communications
dcormack@regenxbio.com
Investors:
George E. MacDougall
Investor Relations
IR@regenxbio.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/regenxbio-initiates-pivotal-phase-of-affinity-duchenne-trial-of-rgx-202-gene-therapy-and-reports-positive-functional-data-302307989.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/regenxbio-initiates-pivotal-phase-of-affinity-duchenne-trial-of-rgx-202-gene-therapy-and-reports-positive-functional-data-302307989.html
SOURCE REGENXBIO Inc.