PureTech Announces Publication of Phase 1 Results for LYT-100 in the Journal Clinical Pharmacology in Drug Development and Provides Timing Updates
LYT-100 well-tolerated at all doses studied with a favorable PK profile; maximum tolerated dose not determined; additional studies underway to evaluate higher doses
Phase 2 enrollment of LYT-100 in patients with Long COVID1 respiratory complications expected to complete by year-end; results anticipated in 1H 2022
Phase 1 healthy volunteer trials underway to further evaluate LYT-100 PK, dosing and tolerability to inform clinical development of LYT-100 across multiple indications; results anticipated in Q1 2022
Today
LYT-100 is PureTech’s wholly-owned therapeutic candidate that is being advanced for the potential treatment of conditions involving inflammation and fibrosis and disorders of lymphatic flow. It is currently being evaluated in two Phase 2 trials in patients with Long COVID respiratory complications and breast cancer-related, upper limb secondary lymphedema. Enrollment in the Long COVID respiratory trial is expected to be completed by the end of 2021, with topline results anticipated in the first half of 2022. Topline results from the breast cancer-related, upper limb secondary lymphedema trial are anticipated in 2022.
“The data set from the completed Phase 1 MAD study, including a favorable safety and tolerability profile, reaffirms our belief that LYT-100 has the potential to be an attractive therapeutic option across a range of conditions. There are substantial shortcomings with the current standards of care for patients living with fibrotic lung disease, and we believe that the anti-fibrotic and anti-inflammatory properties along with the favorable tolerability profile demonstrated with LYT-100 to date could address this issue,” said
LYT-100 is a selectively deuterated form of pirfenidone that retains the pharmacologic properties of the parent compound but is expected to be metabolized at an attenuated rate. GI-related tolerability issues have historically been associated with pirfenidone and have limited its usage in patients at the therapeutic dose approved by the
Multiple ascending dose and food effect study results
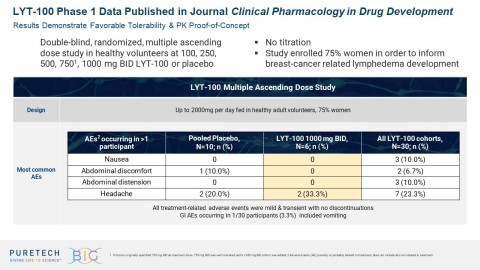
The Phase 1 multiple ascending dose and food effect study was a randomized, double-blind, placebo-controlled study designed to evaluate the safety, tolerability, PK profile and food effect of LYT-100 in healthy volunteers in both fed and fasting states. Plasma concentrations of LYT-100 and its metabolites were measured to determine PK parameters.
Part 1 assessed multiple ascending doses of LYT-100 administered in doses of 100 mg, 250 mg, 500 mg, 750 mg and 1000 mg BID over five days without dose titration. Part 2 assessed the effect of fed versus fasting conditions on the PK profile of LYT-100 following a single 500 mg dose. No dose limiting toxicities were noted, and a maximum tolerated dose was not determined.
All adverse events (AEs) that were possibly or probably related to LYT-100 were mild. Of the 40 participants, 37 (
A dose-proportional PK profile was observed with LYT-100 throughout the range of doses studied. As with pirfenidone, LYT-100 exposure was affected by food, with fed conditions resulting in lower drug exposure compared to fasting conditions. The ratio of exposure during fed conditions was approximately
The therapeutic dose of pirfenidone approved by the FDA for the treatment of IPF is 801 mg three times a day. LYT-100 is designed to potentially improve upon this regimen. In a previously conducted single-dose crossover study, an 801 mg dose of LYT-100 resulted in greater drug exposure than an 801 mg dose of pirfenidone. In part 1 of the multiple ascending dose study, LYT-100 was well-tolerated at a dose above 801 mg.
Additional Phase 1 studies and future development plans
Given that the maximum tolerated dose for LYT-100 was not determined in the original Phase 1 study,
About LYT-100
LYT-100 is PureTech’s most advanced therapeutic candidate from within its Wholly Owned Pipeline. A deuterated form of pirfenidone, an approved anti-inflammatory and anti-fibrotic drug, LYT-100 is being advanced for the potential treatment of conditions involving inflammation and fibrosis, including lung disease (e.g., IPF and potentially other PF-ILDs and Long COVID respiratory complications and related sequelae), and disorders of lymphatic flow, such as lymphedema.
About
For more information, visit www.puretechhealth.com or connect with us on Twitter @puretechh.
Cautionary Note Regarding Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this press release that do not relate to matters of historical fact should be considered forward-looking statements, including without limitation statements that relate to our expectations regarding the potential therapeutic benefit and administration of LYT-100 in patients, including its ability to potentially address certain shortcomings with respect to current standards of care, expectations regarding the clinical development of LYT-100 and the timing for completing enrollment in, or generating data and results from, our current Phase 1 and 2 trials of LYT-100, the potential of clinical data to provide support for further development of LYT-100 across multiple indications, the timing of updates from the Company with respect to future development plans for LYT-100 or other product candidates, our product candidates and approach towards addressing major diseases, and our future prospects, developments, and strategies. The forward-looking statements are based on current expectations and are subject to known and unknown risks, uncertainties and other important factors that could cause actual results, performance and achievements to differ materially from current expectations, including, but not limited to, those risks, uncertainties and other important factors described under the caption “Risk Factors” in our Annual Report on Form 20-F for the year ended
_________________
1 Long COVID is a term being used to describe the emerging and persistent complications following the resolution of COVID-19 infection, also known as post-acute COVID-19 syndrome (PACS).
View source version on businesswire.com: https://www.businesswire.com/news/home/20211116005332/en/
Investors
+1 617 651 3156
amt@puretechhealth.com
+1 774 278 8273
nichole@tenbridgecommunications.com
Source:







