Propanc Biopharma’s Cancer Stem Cell Technology Offers Renewed Hope to Achieve a Total Victory Against Metastatic Cancer
(Photo: Business Wire)
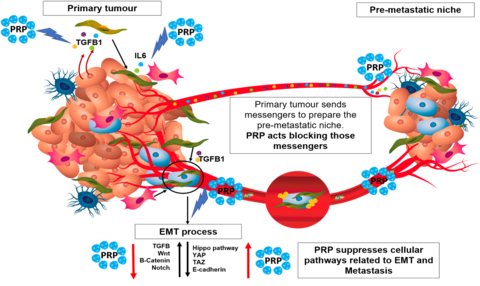
Cancer stem cells are resistant to standard treatments because they can lie dormant for long periods, then migrate to other organs and trigger explosive tumor growth, causing the patient to relapse. Eighty percent of cancers are from solid tumors and metastasis is the main cause of patient death. PRP is designed to target and eradicate cancer stem cells not killed by radiation or chemotherapy. By treating solid tumors with PRP, the tumor loses the ability to generate new cells and the tumor disappears, with no option to form a metastatic tumor elsewhere.
The Company’s scientific researchers have published data confirming that PRP regulates up to 4 relevant pathways, TGFβ, Hippo, Wnt, and Notch, related to cancer spread and metastasis of CSCs. That cascade of reactions disrupts CSC characteristics that leads to tumor invasion into surrounding tissues. PRP interferes with the signals that the primary tumor sends to other tissues to prepare the pre-metastatic niche.
“PRP is a targeted, CSC therapy for the treatment and prevention of metastatic cancer, which I believe can help achieve a total victory in the fight against metastatic cancer from solid tumors,” said Dr
Mr.
About
The Company’s novel proenzyme therapy is based on the science that enzymes stimulate biological reactions in the body, especially enzymes secreted by the pancreas. These pancreatic enzymes could represent the body’s primary defense against cancer.
To view the Company’s “Mechanism of Action” video on its anti-cancer lead product candidate, PRP, please click on the following link: http://www.propanc.com/news-media/video
Forward-Looking Statements
All statements other than statements of historical facts contained in this press release are “forward-looking statements,” which may often, but not always, be identified by the use of such words as “may,” “might,” “will,” “will likely result,” “would,” “should,” “estimate,” “plan,” “project,” “forecast,” “intend,” “expect,” “anticipate,” “believe,” “seek,” “continue,” “target” or the negative of such terms or other similar expressions. These statements involve known and unknown risks, uncertainties and other factors, which may cause actual results, performance or achievements to differ materially from those expressed or implied by such statements. These factors include uncertainties as to the Company’s ability to continue as a going concern absent new debt or equity financings; the Company’s current reliance on substantial debt financing that it is unable to repay in cash; the Company’s ability to successfully remediate material weaknesses in its internal controls; the Company’s ability to reach research and development milestones as planned and within proposed budgets; the Company’s ability to control costs; the Company’s ability to obtain adequate new financing on reasonable terms; the Company’s ability to successfully initiate and complete clinical trials and its ability to successful develop PRP, its lead product candidate; the Company’s ability to obtain and maintain patent protection; the Company’s ability to recruit employees and directors with accounting and finance expertise; the Company’s dependence on third parties for services; the Company’s dependence on key executives; the impact of government regulations, including FDA regulations; the impact of any future litigation; the availability of capital; changes in economic conditions, competition; and other risks, including, but not limited to, those described in the Company’s periodic reports that are filed with the
View source version on businesswire.com: https://www.businesswire.com/news/home/20220302005507/en/
Investor Relations and Media:
Mr.
irteam@propanc.com
+61-3-9882-0780
Source:







