Pluri and Bar-Ilan University to Develop PLX Cells for the Treatment of Cocaine Addiction
- Agreement signed following positive pre-clinical data demonstrating a one-time treatment with PLX-PAD significantly increased neurogenesis, offering immediate, long-term therapeutic effect in treating cocaine addiction
- There are no FDA-approved medications to treat cocaine addiction, a severely unmet need as demand for treatment increases in the U.S. and Europe
- Bar-Ilan University through its tech transfer arm BIRAD Research & Development Company Ltd. receives the right to develop and commercialize the product, Pluri entitled to
20% revenue sharing from future sales of PLX cells as anti-addiction product
HAIFA, Israel, Dec. 21, 2023 (GLOBE NEWSWIRE) -- Pluri Inc. (Nasdaq: PLUR) (TASE: PLUR) (“Pluri” or the “Company”), a leading biotech company that transforms cells into solutions that promote well-being and sustainability, announced it has signed an agreement assigning the joint patent rights to develop Pluri’s PLX cells in the treatment of cocaine addiction, to BIRAD–Research & Development Company Ltd., the commercial arm of Bar-Ilan University. Under the agreement, Bar-Ilan University via BIRAD will receive the right to further develop and commercialize PLX cells as a cocaine anti-addiction product, and Pluri is entitled to
The agreement stems from a collaboration between Pluri and Bar-Ilan University researchers that presented compelling findings. Data from the studies were published in the peer-reviewed journal Pharmaceutics.
PLX-PAD Cells (PAD) attenuate cocaine craving in animal model for drug addiction (level presses indicate the compulsive demand of a rat to inject cocaine intravenous)
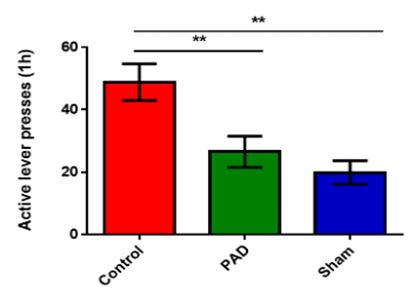
**The control group indicates rats that were trained to inject (lever pressing) cocaine; the sham group was trained to consume a non-addictive substance (saline) instead of cocaine; the PAD group indicates rats trained to inject cocaine that were treated with PAD.
The studies evaluated PLX-PAD cells’ efficacy in treating cocaine addiction in animal models. Findings demonstrate that PLX-PAD cells:
- Reduced cocaine-seeking behavior by migrating to specific mesolimbic regions of the brain and restoring neurogenesis
- Significantly increased neurogenesis
- Decreased cocaine cravings during withdrawal
- Significantly decreased drug cravings following drug relapse at 14 days and 28 days following treatment
- PLX Cells remained detectable in addiction-related brain areas 28 days post-injection
Prof. Gal Yadid of The Leslie and Susan Gonda Multidisciplinary Brain Research Center of Bar-Ilan University, a leading neuropsychopharmacologist focused on developing novel neuropsychiatric therapeutics for various indications such as addictions, depression, PTSD and more, including peptide drugs, microbiotics and others commented, “Based on several models, PLX offers great hope as a healing modality for the treatment of cocaine addiction as compared to traditional pharmacological methods that only address symptoms such as depression and anxiety, resulting in high relapse rate to drug usage even after long time of detoxification. The data suggest PLX offers a noninvasive one-time treatment that produces an immediate, long-term effect. We look forward to further exploring PLX’s mechanism of action, which we believe is likely through the cells’ migration to specific mesolimbic regions of the brain, thereby improving the regions’ plasticity by restoring neurons in the hippocampus.”
“PLX cells showed great data to act as a potential treatment for the millions of people around the world who suffer from cocaine addiction,” said Pluri Chief Executive Officer and President Yaky Yanay. “Pluri’s collaboration with BIRAD and Bar-Ilan is a great example of our co-development strategy, utilizing our advanced technology together with partners to develop life-changing products.”
In the U.S. alone, 1.2 million people suffered from cocaine use disorder in 2021, and approximately 24,500 people died from an overdose involving cocaine. Across North America and Europe, demand for treatment has steadily increased over the last decade, according to the U.N. Office on Drugs and Crime.
About BIRAD
BIRAD – Research & Development Company Ltd. was established in order to translate new inventions made at Bar-Ilan University into useful products that can be effectively commercialized, thus strengthening the economy, promoting innovation and improving lives.
BIRAD's innovative approach, combined with Bar-Ilan University’s rapid growth leading Israel's growth in students' number, including the largest Nanotechnology center in Israel and new Medical School in Safed, provides BIRAD with a wide range of opportunities. Thus, BIRAD offer corporate partnerships and alliances, intellectual property management, and technology commercialization through venture creation and licensing.
About PLX-PAD
PLX-PAD cells have a “nose” that can recognize pathological tissue and navigate directly to this target, home in and release their cargo. Hence, they exhibit regenerative potential due to their capacity to release specific loaded factors in response to distress signals from tissues that have been damaged by muscle trauma, ischemia, or inflammation. These factors harness the body’s repair mechanisms to support tissue regeneration and differentiation. PLX-PAD cells also exhibit immune-modulating capabilities, playing a central role in the body’s response to tissue injury.
About Pluri Inc.
Pluri is pushing the boundaries of science and engineering to create cell-based products for commercial use and is pioneering a biotech revolution that promotes global well-being and sustainability. The Company’s technology platform, a patented and validated state-of-the-art 3D cell expansion system, advances novel cell-based solutions for a range of initiatives — from medicine and climate change to food scarcity, animal cruelty, and beyond. Pluri’s method is uniquely accurate, scalable, cost-effective, and consistent from batch to batch. Pluri currently operates in the field of regenerative medicine, food-tech, and biologics and aims to establish partnerships that leverage the Company’s 3D cell-based technology in additional industries that require effective, mass cell production. To learn more, visit us at pluri-biotech.com or follow us on LinkedIn and X (formerly known as Twitter).
Safe Harbor Statement
This press release contains express or implied forward-looking statements within the Private Securities Litigation Reform Act of 1995 and other U.S. Federal securities laws. For example, Pluri uses forward-looking statements when it discusses the potential of PLX-PAD cells to treat people with cocaine addictions. These forward-looking statements and their implications are based on the current expectations of the management of Pluri only and are subject to a number of factors and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements. The following factors, among others, could cause actual results to differ materially from those described in the forward-looking statements about Pluri: changes in technology and market requirements; Pluri may encounter delays or obstacles in launching and/or successfully completing its clinical trials, if necessary; its products may not be approved by regulatory agencies, its technology may not be validated as it progresses further and its methods may not be accepted by the scientific community; it may be unable to retain or attract key employees whose knowledge is essential to the development of its products; unforeseen scientific difficulties may develop with its processes; its products may wind up being more expensive than it anticipates; results in the laboratory may not translate to equally good results in real clinical settings; its patents may not be sufficient; its products may harm recipients or consumers; changes in legislation with an adverse impact; inability to develop and introduce new technologies in a timely fashion, products and applications; loss of market share and pressure on pricing resulting from competition, which could cause the actual results or performance of Pluri to differ materially from those contemplated in such forward-looking statements. Except as otherwise required by law, Pluri undertakes no obligation to publicly release any revisions to these forward-looking statements to reflect events or circumstances after the date hereof or to reflect the occurrence of unanticipated events. For a more detailed description of the risks and uncertainties affecting Pluri, reference is made to Pluri's reports filed from time to time with the Securities and Exchange Commission.
Pluri Contacts
Investors: investor.relations@pluri-biotech.com
Israel Media: Shachar Yental at shacharye@gitam.co.il
U.S. Media: Nathan Miller at nathan@miller-ink.com / Brianna Ziegler at brianna@miller-ink.com
Bar-Ilan University Contact
Anat Lev - Confortes: anat.lev1@biu.ac.il
A graph accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/2896e248-f7d2-4866-a943-3f04343b9ed9







