Precigen Announces Positive Interim Phase 1 Data for PRGN-3006 UltraCAR-T® in Relapsed or Refractory Acute Myeloid Leukemia
Precigen presented promising interim results for its PRGN-3006 UltraCAR-T therapy targeting relapsed or refractory acute myeloid leukemia (AML) at the 63rd ASH Annual Meeting. The Phase 1/1b study included 15 patients, showing an overall response rate (ORR) of 50% in the lymphodepletion cohort. Notably, the therapy was well-tolerated, with no dose-limiting toxicities or neurotoxicity. The company anticipates progressing to a multicenter expansion phase to evaluate repeated dosing. These results highlight the potential of UltraCAR-T in transforming AML treatment pathways.
- Overall response rate of 50% in lymphodepletion cohort.
- No dose-limiting toxicities or neurotoxicity reported.
- Excellent dose-dependent expansion of UltraCAR-T cells.
- Successful detection of UltraCAR-T cells for over 7 months post-infusion.
- None.
Insights
Analyzing...
GERMANTOWN, Md., Dec. 13, 2021 /PRNewswire/ -- Precigen, Inc., a biopharmaceutical company specializing in the development of innovative gene and cell therapies to improve the lives of patients, today presented positive interim data at the 63rd ASH Annual Meeting and Exposition (Abstract# 825) from the ongoing Phase 1/1b clinical study of PRGN-3006 UltraCAR-T® in patients with relapsed or refractory (r/r) acute myeloid leukemia (AML) and higher risk myelodysplastic syndromes (MDS) (clinical trial identifier: NCT03927261). The oral presentation was delivered by David Sallman, MD, Assistant Member in the Department of Malignant Hematology at the H. Lee Moffitt Cancer Center & Research Institute (Moffitt) and a lead investigator for the PRGN-3006 clinical trial.
PRGN-3006 UltraCAR-T is a multigenic autologous CAR-T simultaneously expressing a CAR specifically targeting CD33; membrane bound IL-15 (mbIL15) for enhanced in vivo expansion and persistence; and a kill switch to conditionally eliminate CAR-T cells for an improved safety profile. CD33 is over-expressed on AML blasts with lesser expression on normal hematopoietic stem cells. PRGN-3006 UltraCAR-T has been granted Orphan Drug Designation in patients with AML by the US Food and Drug Administration (US FDA).
The Phase 1/1b clinical study is designed to enroll in two phases, an initial dose escalation phase followed by a dose expansion phase, to evaluate safety and determine the maximum tolerated dose of PRGN-3006 delivered via intravenous (IV) infusion without lymphodepletion (Cohort 1) or with lymphodepletion (Cohort 2). The study is also evaluating in vivo persistence and anti-tumor activity of PRGN-3006.
Today's ASH presentation included data from 15 r/r AML patients treated in the non-lymphodepletion cohort (N=9) and the lymphodepletion cohort (N=6). Patients were heavily pre-treated with a median of 4 (range: 1 to 6) and 3 (range: 1 to 7) prior regimens in the non-lymphodepletion and the lymphodepletion cohorts, respectively. Additionally,
Safety Data
PRGN-3006 was well-tolerated with no dose-limiting toxicities (DLTs) and no neurotoxicity at any dose level. Overall, there was low incidence of adverse events following PRGN-3006 infusion and the most common adverse events were decreased lymphocyte count, anemia and cytokine release syndrome (CRS). More than
Clinical Activity
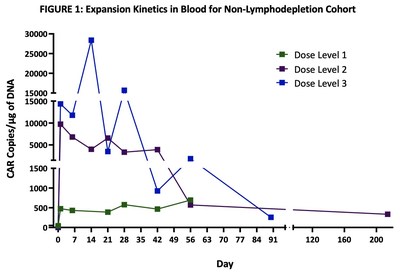
Non-lymphodepletion Cohort
Excellent dose-dependent expansion and persistence of PRGN-3006 in peripheral blood and bone marrow was observed following a single infusion, with detection of UltraCAR-T cells in blood for more than 7 months post-infusion highlighting the ability of UltraCAR-T cells to engraft and survive even in the absence of lymphodepletion. Peak expansion was observed between days 7 and 21 in the peripheral blood (FIGURE 1).
In the non-lymphodepletion cohort at the three dose levels evaluated, 3 out of 9 (
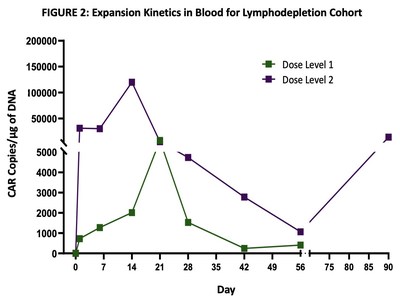
Lymphodepletion Cohort
Excellent dose-dependent expansion and persistence of PRGN-3006 in peripheral blood and bone marrow was observed following a single infusion, with detection of UltraCAR-T cells in blood for more than 3 months post-infusion. Peak expansion was observed between days 14 and 21 in the peripheral blood with higher peak expansion (> 10 fold) observed in the lymphodepletion cohort (FIGURE 2) at the same dose level.
An ORR of
TABLE 1: Summary Objective Response Data for the Lymphodepletion Cohort
Dose Level | AML Subtype | Dose Received | Age | Sex | Prior | Safety** | Objective Response*** |
DL 1 | Persistent AML | 8.7 x 106 | 60 | F | 2 prior: CLAG and HiDAC | No incidence | CRh at Day 84 |
DL 2 | Extramedullary AML | 28 x 106 | 53 | M | 7 prior: intensive chemo, | No incidence | PR# |
AML | 20 x 106 | 61 | F | 4 prior: vyxeos, HMA+venetoclax, | CRS Grade 1, | CRi at Day 28 CRh at Day 60 |
*CLAG=cladribine, cytarabine, and granulocyte-stimulating factor; HiDAC=high-dose cytarabine; FLAG=fludarabine, cytarabine and filgrastim; anti-IDH1=isocitrate dehydrogenases 1 inhibitor; HMA=hypomethylating agents (HMA); allo-HSCT= allogeneic hematopoietic stem cell transplant |
Analysis of peripheral blood samples post PRGN-3006 infusion showed gene expression changes consistent with improvement in the immune compartment function for anti-tumor effect in responders. There was an increase in cytotoxicity, costimulatory signaling, and lymphoid compartment and decreased apoptosis pathway scores in the lymphodepletion cohort on Days 14 and 28 post PRGN-3006 treatment compared to baseline.
The study is anticipated to progress to the multicenter expansion phase with the plan to evaluate the potential of repeated dosing of PRGN-3006.
"The interim data for PRGN-3006 showed excellent, dose-dependent expansion and persistence of PRGN-3006 in peripheral blood and bone marrow following a single infusion, with detection of UltraCAR-T cells in blood more than 3 months post-infusion in the non-lymphodepletion and lymphodepletion cohorts," said David A. Sallman, MD, of Moffitt and lead investigator for the PRGN-3006 clinical study. "An ORR of
"We are excited by these interim data, which clearly highlight the extraordinary potential and flexibility of the UltraCAR-T platform to deliver precision medicine to patients at any time, at any place and as many times as needed," said Helen Sabzevari, PhD, President and CEO of Precigen. "Based on the favorable safety profile and excellent expansion observed for both the lymphodepletion and the non-lymphodepletion cohorts, we believe UltraCAR-T cells have the potential to improve outcomes for cancer patients."
Precigen: Advancing Medicine with Precision™
Precigen (Nasdaq: PGEN) is a dedicated discovery and clinical stage biopharmaceutical company advancing the next generation of gene and cell therapies using precision technology to target the most urgent and intractable diseases in our core therapeutic areas of immuno-oncology, autoimmune disorders, and infectious diseases. Our technologies enable us to find innovative solutions for affordable biotherapeutics in a controlled manner. Precigen operates as an innovation engine progressing a preclinical and clinical pipeline of well-differentiated unique therapies toward clinical proof-of-concept and commercialization. For more information about Precigen, visit www.precigen.com or follow us on Twitter @Precigen, LinkedIn or YouTube.
About Acute Myeloid Leukemia (AML)
AML is a cancer that starts in the bone marrow, but most often moves into the blood.1 Though considered rare, AML is among the most common types of leukemia in adults.2 In 2019, it was estimated that 21,450 new cases of AML would be diagnosed in the US.2 AML is uncommon before the age of 45 and the average age of diagnosis is about 68.2 The prognosis for patients with AML is poor with an average 5–year survival rate of approximately 25 percent overall, and less than a 5 percent 5–year survival rate for patients older than 65.3 Amongst elderly AML patients (≥ 65 years of age), median survival is short, ranging from 3.5 months for patients 65 to 74 years of age to 1.4 months for patients ≥ 85 years of age.3
About Myelodysplastic Syndrome (MDS)
MDS are diseases of the bone marrow generally found in adults in their 70s.4 Incidence in the US is not known for sure, but estimates range from 10,000 each year and higher.4 Using International Prognostic Scoring System (IPSS-R), median survival for MDS patients can vary from less than one year for the "very high" IPSS-R risk group to more than eight years for the "very low" IPSS-R group.4
UltraCAR-T®
UltraCAR-T is a multigenic autologous CAR-T platform that utilizes Precigen's advanced non-viral Sleeping Beauty system to simultaneously express an antigen-specific CAR to specifically target tumor cells, mbIL15 for enhanced in vivo expansion and persistence, and a kill switch to conditionally eliminate CAR-T cells for a potentially improved safety profile. Precigen has advanced the UltraCAR-T platform to address the inhibitory tumor microenvironment by incorporating a novel mechanism for intrinsic checkpoint blockade without the need for complex and expensive gene editing techniques. UltraCAR-T investigational therapies are manufactured via Precigen's overnight manufacturing process using the proprietary UltraPorator electroporation system at the medical center and administered to patients only one day following gene transfer. The overnight UltraCAR-T manufacturing process does not use viral vectors and does not require ex vivo activation and expansion of T cells, potentially addressing major limitations of current T cell therapies.
UltraPorator™
The UltraPorator system is an exclusive device and proprietary software solution for the scale-up of rapid and cost-effective manufacturing of UltraCAR-T therapies and potentially represents a major advancement over current electroporation devices by significantly reducing the processing time and contamination risk. The UltraPorator device is a high-throughput, semi-closed electroporation system for modifying T cells using Precigen's proprietary non-viral gene transfer technology. UltraPorator is being utilized for clinical manufacturing of Precigen's investigational UltraCAR-T therapies in compliance with current good manufacturing practices.
Trademarks
Precigen, UltraCAR-T, UltraPorator and Advancing Medicine with Precision are trademarks of Precigen and/or its affiliates. Other names may be trademarks of their respective owners.
Cautionary Statement Regarding Forward-Looking Statements
Some of the statements made in this press release are forward-looking statements. These forward-looking statements are based upon the Company's current expectations and projections about future events and generally relate to plans, objectives, and expectations for the development of the Company's business, including the timing and progress of preclinical studies, clinical trials, discovery programs and related milestones, the promise of the Company's portfolio of therapies, and in particular its CAR-T and AdenoVerse therapies. Although management believes that the plans and objectives reflected in or suggested by these forward-looking statements are reasonable, all forward-looking statements involve risks and uncertainties, including the possibility that the timeline for the Company's clinical trials might be impacted by the COVID-19 pandemic, and actual future results may be materially different from the plans, objectives and expectations expressed in this press release. The Company has no obligation to provide any updates to these forward-looking statements even if its expectations change. All forward-looking statements are expressly qualified in their entirety by this cautionary statement. For further information on potential risks and uncertainties, and other important factors, any of which could cause the Company's actual results to differ from those contained in the forward-looking statements, see the section entitled "Risk Factors" in the Company's most recent Annual Report on Form 10-K and subsequent reports filed with the Securities and Exchange Commission.
References |
1 American Cancer Society. What is Acute Myeloid Leukemia (AML)? |
2 American Cancer Society. Key Statistics for Acute Myeloid Leukemia (AML) |
3 Thein, M., et al., Outcome of older patients with acute myeloid leukemia: an analysis of SEER data over 3 decades. Cancer, 2013. 119(15): p.2720-7 |
4 American Cancer Society. Key Statistics for Myelodysplastic Syndromes |
Investor Contact:
Steven Harasym
Vice President, Investor Relations
Tel: +1 (301) 556-9850
investors@precigen.com
Media Contacts:
Donelle M. Gregory
press@precigen.com
Glenn Silver
Lazar-FINN Partners
glenn.silver@finnpartners.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/precigen-announces-positive-interim-phase-1-data-for-prgn-3006-ultracar-t-in-relapsed-or-refractory-acute-myeloid-leukemia-301443455.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/precigen-announces-positive-interim-phase-1-data-for-prgn-3006-ultracar-t-in-relapsed-or-refractory-acute-myeloid-leukemia-301443455.html
SOURCE Precigen, Inc.