NRx Pharmaceuticals (NASDAQ:NRXP) Reports Second Quarter and Year to Date 2024 Financial Results and Provides Business Update
NRx Pharmaceuticals (NASDAQ:NRXP) reported Q2 2024 financial results and provided a business update. Key highlights include:
1. Secured $10.8-$16.3 million in convertible-debt funding to support FDA New Drug Applications for NRX-100 (ketamine) and NRX-101.
2. Plans to file NDAs for NRX-100 in suicidal depression and NRX-101 for Accelerated Approval in bipolar depression with akathisia or suicidality.
3. Reduced net loss to $7.9 million in Q2 2024, down from $8.7 million in Q2 2023.
4. Decreased R&D expenses to $2.8 million in Q2 2024 from $3.9 million in Q2 2023.
5. HOPE Therapeutics subsidiary focused on ketamine clinics planned for spinout.
NRx Pharmaceuticals (NASDAQ:NRXP) ha riportato i risultati finanziari per il secondo trimestre del 2024 e fornito un aggiornamento sulla propria attività. I principali punti salienti includono:
1. Sicurezza di finanziamenti in debito convertibile da $10.8 a $16.3 milioni per sostenere le domande di nuovi farmaci (NDA) per NRX-100 (ketamina) e NRX-101.
2. Pianificazione di presentare NDA per NRX-100 per la depressione suicidaria e NRX-101 per approvazione accelerata nella depressione bipolare con akathisia o suicidialità.
3. Riduzione della perdita netta a $7.9 milioni nel secondo trimestre del 2024, rispetto ai $8.7 milioni del secondo trimestre del 2023.
4. Diminuzione delle spese per R&D a $2.8 milioni nel secondo trimestre del 2024 rispetto ai $3.9 milioni del secondo trimestre del 2023.
5. La controllata HOPE Therapeutics, focalizzata su cliniche di ketamina, è pianificata per un'uscita autonoma.
NRx Pharmaceuticals (NASDAQ:NRXP) reportó los resultados financieros del segundo trimestre de 2024 y proporcionó una actualización sobre sus negocios. Los aspectos más destacados incluyen:
1. Aseguró financiamiento en deuda convertible entre $10.8 y $16.3 millones para respaldar las solicitudes de nuevos medicamentos (NDA) para NRX-100 (ketamina) y NRX-101.
2. Planes para presentar NDAs para NRX-100 en depresión suicida y NRX-101 para aprobación acelerada en depresión bipolar con akatisia o suicidialidad.
3. Reducción de la pérdida neta a $7.9 millones en el segundo trimestre de 2024, frente a los $8.7 millones del segundo trimestre de 2023.
4. Disminución de los gastos en I+D a $2.8 millones en el segundo trimestre de 2024 desde $3.9 millones en el segundo trimestre de 2023.
5. La subsidiaria HOPE Therapeutics, enfocada en clínicas de ketamina, está planeada para escindirse.
NRx Pharmaceuticals (NASDAQ:NRXP)는 2024년 2분기 재무 결과를 발표하고 사업 업데이트를 제공했습니다. 주요 하이라이트는 다음과 같습니다:
1. NRX-100 (케타민)과 NRX-101에 대한 FDA 신규 의약품 신청을 지원하기 위해 $10.8~$16.3백만의 전환사채 자금을 확보했습니다.
2. 자살 우울증에 대한 NRX-100와 비약물적 동기 부여가 있는 이중 우울증에 대한 NRX-101의 가속 승인 신청서를 제출할 계획입니다.
3. 2024년 2분기 순손실을 $7.9백만으로 줄였으며, 이는 2023년 2분기의 $8.7백만에서 감소한 수치입니다.
4. 2024년 2분기 연구개발(R&D) 비용을 $2.8백만으로 줄였으며, 이는 2023년 2분기의 $3.9백만에서 감소한 것입니다.
5. 케타민 클리닉에 집중하는 HOPE Therapeutics 자회사가 분사 예정입니다.
NRx Pharmaceuticals (NASDAQ:NRXP) a publié les résultats financiers du deuxième trimestre 2024 et a fourni une mise à jour sur son activité. Les principaux points à retenir comprennent :
1. Obtention d'un financement en dette convertible de 10,8 à 16,3 millions de dollars pour soutenir les demandes de nouveaux médicaments (NDA) pour NRX-100 (kétamine) et NRX-101.
2. Prévisions de soumettre des NDA pour NRX-100 dans la dépression suicidaire et NRX-101 pour une approbation accélérée dans la dépression bipolaire avec akathisie ou tendance suicidaire.
3. Réduction de la perte nette à 7,9 millions de dollars au deuxième trimestre 2024, en baisse par rapport à 8,7 millions de dollars au deuxième trimestre 2023.
4. Diminution des dépenses en R&D à 2,8 millions de dollars au deuxième trimestre 2024 contre 3,9 millions de dollars au deuxième trimestre 2023.
5. La filiale HOPE Therapeutics, spécialisée dans les cliniques de kétamine, est prévue pour être scindée.
NRx Pharmaceuticals (NASDAQ:NRXP) berichtete über die Finanzergebnisse des 2. Quartals 2024 und gab ein Update zur Geschäftslage. Zu den wichtigsten Punkten gehören:
1. Sicherstellung von 10,8 bis 16,3 Millionen US-Dollar an wandelbaren Schulden zur Unterstützung von FDA-Anträgen für neue Arzneimittel (NDA) für NRX-100 (Ketamin) und NRX-101.
2. Geplante Einreichung von NDAs für NRX-100 bei suizidaler Depression und NRX-101 für beschleunigte Genehmigung bei bipolaren Depressionen mit Akathisie oder Suizidalität.
3. Reduzierung des Nettoverlusts auf 7,9 Millionen US-Dollar im 2. Quartal 2024, ein Rückgang von 8,7 Millionen US-Dollar im 2. Quartal 2023.
4. Senkung der F&E-Ausgaben auf 2,8 Millionen US-Dollar im 2. Quartal 2024 von 3,9 Millionen US-Dollar im 2. Quartal 2023.
5. Die Tochtergesellschaft HOPE Therapeutics, die sich auf Ketamin-Kliniken konzentriert, plant eine Abspaltung.
- Secured $10.8-$16.3 million in convertible-debt funding with improved terms
- Reduced net loss by 10% year-over-year in Q2 2024
- Decreased R&D expenses by $1.1 million in Q2 2024 compared to Q2 2023
- Improved net loss per share by 47% for the first six months of 2024 compared to 2023
- Progressing towards NDA filings for NRX-100 and NRX-101
- Cash and cash equivalents of only $1.9 million as of June 30, 2024
- Slight increase in general and administrative expenses in Q2 2024
- Ongoing need for additional funding to support operations and NDA filings
Insights
NRx Pharmaceuticals' Q2 2024 results show a 10% reduction in net loss to
The company's focus on two potential NDAs for NRX-100 and NRX-101 could be game-changing if approved. The planned spin-out of HOPE Therapeutics could also unlock value for shareholders. However, with only
NRx's progress with NRX-100 (ketamine) for suicidal depression is promising, backed by data from four clinical trials involving nearly 1,000 participants. The recent 43,000-person cohort study confirming ketamine's efficacy adds substantial weight to their NDA filing. For NRX-101, the Phase 2b/3 trial results in suicidal bipolar depression patients are encouraging, showing comparable efficacy to standard care and significant reductions in akathisia and time to sustained remission from suicidality.
The company's unique preservative-free ketamine formulation and pH-neutral version could offer competitive advantages. The potential expansion into chronic pain and antibiotic-resistant UTI treatment with NRX-101 demonstrates versatility in their pipeline. However, success hinges on FDA approvals and market adoption, which remain uncertain despite promising data.
- Company is now funded for and focused on New Drug Applications (NDAs) for NRX-100 (ketamine) and NRX-101
- Audit of HOPE Therapeutics is now complete, SEC filing of spinout this quarter
Key Milestones
- Secured
$10.8 $16.3 million - Retirement of Streeterville debt and settlement of litigation at a substantial discount to litigation claims
- NRX-100 NDA for suicidal depression based on data from four clinical trials in nearly 1000 participants demonstrating highly significant efficacy compared to placebo, active comparator, and electroshock therapy
- Ketamine findings have just been confirmed in published 43,000 person cohort study1
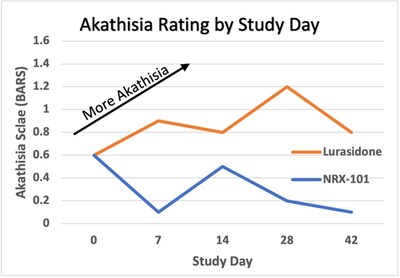
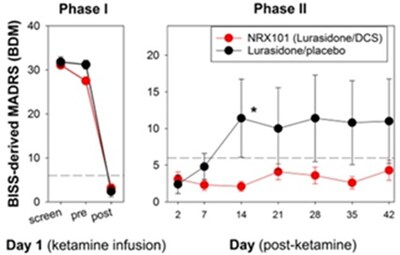
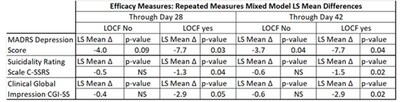
- Phase 2b/3 trial of NRX-101 in suicidal patients with bipolar depression demonstrated depression efficacy comparable to standard of care and significant reduction of akathisia (P=0.025) and time to sustained remission from suicidality (P=.05). Presented at the annual meeting of the American Society of Clinical Psychopharmacology. Profile demonstrates possible best in class bipolar depression medication
- Company plans to file a New Drug Application (NDA) for Accelerated Approval under Breakthrough Therapy Designation and Priority Review of NRX-101 in treatment of bipolar depression in people akathisia or suicidality, based on the Phase 2b/3 and STABIL-B data
- Stability data continues to mature on the three manufacturing lots required for the NRX-100 (IV ketamine) NDA filing and the Company announced alignment with FDA on its Pediatric Study Plan for NRX-100, also a requirement for filing an NDA
- HOPE Therapeutics, the Company's wholly owned subsidiary, is focused on developing a best-in-class network of clinics that currently offer ketamine and other lifesaving therapies to patients with suicidal depression and related disorders. HOPE is planned to be spun out as a separate company to be owned by NRx, current NRx shareholders, and new investors. This effort will be funded apart from NRx.
- Appointed Dr. Dennis McBride, a Neuroscience, Information Technology and Medical Technology Veteran, to its Board of Directors
- Management to host a conference call August 14, 2024, at 4:30 PM ET
"NRx has continued to execute on our plans to file two NDA's this year. As we near maturation of NRX-100 stability data, we also achieved an important milestone in aligning with FDA on our pediatric study plan. Together with strong clinical data from four clinical trials, we believe this application will be quite robust. Additionally, the important data generated from two trials conducted by NRx with NRX-101 in suicidal bipolar depression sets the stage for a second NDA for Accelerated Approval later this year. Finally, work continues to spin out Hope Therapeutics and distribute shares to NRx stockholders. We believe reaching these important milestones will generate significant value in the company and reward our shareholders," said Jonathan Javitt, MD, MPH, Chairman and Chief Scientist of NRx Pharmaceuticals. "Facilitated by funding to allow us to achieve these goals and while replacing the prior expensive and toxic debt on our balance sheet, we are in a position to deliver therapy that can meet considerable unmet medical need in millions of patients across the country. We are dedicated to bringing hope to life and I thank our team and shareholders for their ongoing hard work and support."
Second Quarter Clinical, Regulatory and Corporate Highlights
Funding for FDA filings of NRX-100 and NRX-101
The Company has executed a Convertible Debt instrument with Anson Funds of
Retirement of current debt and settlement of litigation
Concurrent with the Anson investment, the Company has settled its outstanding litigation with Streeterville Capital, LLC at a substantial discount to the amounts claimed in litigation.
Progress towards an NDA for NRX-100 (IV ketamine) in the treatment of suicidal depression
Intravenous ketamine has now become a standard of care for acute treatment of suicidal depression, in the absence of an FDA-labeled product. Intranasal Esketamine is approved by the FDA (SPRAVATO®) but has not demonstrated a benefit on suicidality and is not approved for use in patients with bipolar depression. Attempts to use intranasal racemic ketamine for suicidal depression have failed.
The Company has formed data-sharing partnerships to license clinical trial data from a French Government-funded trial and two NIH-funded trials all of which demonstrate efficacy of racemic Intravenous ketamine against depression and two of which demonstrate statistically significant benefit vs suicidality. The Company's role is to reformat these data into the required presentation required for review by the FDA.
In contrast to nasal ketamine, Intravenous racemic ketamine demonstrates dramatic and immediate reduction of suicidality in patients with both Major Depressive Disorder and Bipolar Depression. Grunebaum and colleagues demonstrated a rapid and statistically significant reduction in Suicidal Ideation (SSI) at day 1 (p=0.0003) and in depression (P=0.0234), as measured by the Profile of Mood States (POMS) among patients randomized to IV Ketamine compared to those randomized to midazolam. This trial was published in the American Journal of Psychiatry Grunebaum, et.al.2. Abbar and colleagues similarly published
In November 2023, the Company initiated manufacture of ketamine together with Nephron Pharmaceuticals, Inc. (
A long-term challenge with ketamine is that the current formulation (KETALAR®) is highly acidic. While it is suitable for intravenous use, it cannot be administered subcutaneously. In March 2024 the Company demonstrated the formulation of a pH neutral patentable form of IV ketamine that it anticipates will have widespread applicability both in treatment of depression and chronic pain.
Development of NRX-101 for Suicidal Treatment-Resistant Suicidal Bipolar Depression
The Company presented final data from the recently completed phase 2b/3 trial of NRX-101 in suicidal bipolar depression4 at the American Society of Clinical Psychopharmacology's annual meeting. These data demonstrated a significantly improved safety profile versus the standard of care, as demonstrated by a clinically significant reduction in akathisia (P=0.025) and time to sustained remission from suicidality (P=0.05). Akathisia is an adverse event seen with antidepressant medications considered by many experts to be a precursor to suicide. Given the vital need for safer medications in this at-risk population, we plan to submit an NDA for Accelerated Approval to the US FDA for treatment of bipolar depression patients with suicidality or akathisia, based on these data as well as additional data from our STABIL-B5 trial.
Incorporation of HOPE Therapeutics
In February we first presented the contours of HOPE Therapeutics, a subsidiary that will focus on the delivery of advance psychiatric treatments, including ketamine-focused treatment for depression and suicidality. Unlike the core business of NRx Pharmaceuticals, that is focused on biotechnology Research and Development, HOPE is organized around consolidating existing best-in-class clinics into a nationwide network. This has been done previously and without much success with clinics that are not necessarily psychiatrist-led. As currently designed, the HOPE consolidation is likely to be funded as a separate entity, through bond offerings and, thus, to be non-dilutive to NRx shareholders. Over the past quarter, HOPE leadership has identified the clinics that are most likely to participate in the first
With Hope ownership as an asset of NRx, this will further strengthen the NRx balance sheet and aims to further enhance NRx shareholder value.
NRX-101 for Treatment of Chronic Pain:
In 2023, the Company licensed US Patent 8,653,120 for the use of DCS in chronic pain and filed a now-accepted Investigational New Drug (IND) application with the FDA to initiate commercial drug development of NRX-101 in chronic pain. Data lock has now been achieved in a 200-person randomized prospective trial funded by the US DOD (NCT 03535688) in which patients with chronic pain were randomly assigned to DCS 400mg/day vs. placebo. Should these results support efficacy of DCS in the treatment of chronic low back pain, they are expected to provide a Breakthrough Therapy path towards treatment of chronic pain with DCS and DCS-containing medicines.
Treatment of Urinary Tract Infection (UTI) and Urosepsis:
Although treatment of UTI is quite different from use of NRX-101 to treat Central Nervous System disorders, D-cycloserine was originally developed as an antibiotic because of its role in disrupting the cell wall of certain pathogens. During Q3 2023, NRx tested NRX-101 and its components against resistant pathogens that appear on the Congressionally mandated Qualified Infectious Disease Product (QIDP) list and proved in vitro effectiveness against antibiotic-resistant E. coli, Pseudomonas, and Acinetobacter. Accordingly, NRx was granted QIDP designation, Fast Track Designation, and Priority Review by the US FDA in January 2024.
In recent years, increased antibiotic resistance to common pathogens that cause urinary tract infections and urosepsis (i.e., sepsis originating in the urinary tract) has resulted in a marked increase in cUTI, hospitalization, and death from urosepsis. The US Center for Disease Control and Prevention reports that more than 1.7 million Americans contract sepsis each year, of whom at least 350,000 die during their hospitalization or are discharged to hospice (CDC Sepsis Ref.)6. There are approximately 3 million patients per year who contract cUTI in the US annually (Lodise, et. al.)7. Additionally, should NRX-101 succeed in clinical trials, the Company will consider developing a follow-on product that is anticipated to achieve another 20 years of patent exclusivity.
A key challenge in the treatment of cUTI is the tendency of advanced antibiotics to cause C. difficile infection, which is fatal in
In The Company does not anticipate funding this initiative with core NRx assets and is exploring structures for partnership opportunities. Should the Company or its partners succeed in serving
Financial Results for the Quarter and Year to Date 2024
For the three months ended June 30, 2024, we at NRx Pharmaceuticals reduced our net loss from
For the six months ended June 30, 2024, NRx Pharmaceuticals reduced its net loss to
As of June 30, 2024, we had
NRx continues to implement operational efficiencies to extend cash runway and maintain focus on our path to generating revenue and value for our shareholders.
Please see detailed financials on our Form 10-Q, filed with the SEC and available on our website.
Conference Call and Webcast Details
A live webcast of the conference call will be available on the Company's website at 4:30 p.m. ET today, at https://ir.nrxpharma.com/events. An archive of the webcast will be available on the Company's website for 30 days. Participants that are unable to join the webcast can access the conference call via telephone by dialing domestically 1-800-717-1738 or internationally 1-646-307-1865.
About NRx Pharmaceuticals, Inc.
NRx Pharmaceuticals is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain, and PTSD. The Company is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain. NRx plans to file an NDA for Accelerated Approval for NRX-101 in patients with bipolar depression and suicidality or akathisia. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRx has recently announced plans to submit a New Drug Application for NRX-100 (IV ketamine) for the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRx was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
About HOPE Therapeutics, Inc.
HOPE Therapeutics, Inc. (www.hopetherapeutics.com) is a care delivery company developing a best-in-class network of clinics that currently offer ketamine and other lifesaving therapies to patients with suicidal depression and related disorders, together with a digital therapeutic-enabled platform designed to augment and preserve the clinical benefit of NMDA-targeted drug therapy.
Notice Regarding Forward-Looking Statements
The information contained herein includes forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and Section 27A of the Securities Act of 1933, as amended. These statements include, among others, statements regarding the proposed public offering and the timing and the use of the proceeds from the offering. Forward-looking statements generally include statements that are predictive in nature and depend upon or refer to future events or conditions, and include words such as "may," "will," "should," "would," "expect," "plan," "believe," "intend," "look forward," and other similar expressions among others. These statements relate to future events or to the Company's future financial performance, and involve known and unknown risks, uncertainties and other factors that may cause the Company's actual results to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. You should not place undue reliance on forward-looking statements since they involve known and unknown risks, uncertainties and other factors which are, in some cases, beyond the Company's control and which could, and likely will, materially affect actual results, levels of activity, performance or achievements. Any forward-looking statement reflects the Company's current views with respect to future events and is subject to these and other risks, uncertainties and assumptions relating to the Company's operations, results of operations, growth strategy and liquidity. More detailed information about the Company and the risk factors that may affect the realization of forward-looking statements is set forth in the Company's most recent Annual Report on Form 10-K and other filings with the Securities and Exchange Commission. Investors and security holders are urged to read these documents free of charge on the SEC's website at http://www.sec.gov. Except as may be required by applicable law, The Company assumes no obligation to publicly update or revise these forward-looking statements for any reason, or to update the reasons actual results could differ materially from those anticipated in these forward-looking statements, whether as a result of new information, future events or otherwise.
1 Pan, Y., Gorenflo, M.P., Davis, P.B. et al. Suicidal ideation following ketamine prescription in patients with recurrent major depressive disorder: a nation-wide cohort study. Transl Psychiatry 14, 327 (2024). https://doi.org/10.1038/s41398-024-03033-4
2 Grunebaum, et. al., Ketamine for Rapid Reduction of Suicidal Thoughts. Am J Psychiatry. 2018 Apr 1: 175(4): 327-335
3 Abbar, et. al. Ketamine for Acute Treatment of Severe Suicidal Ideation, BMJ 2022; 376
4 Nierenberg, et. al., A Randomized, Double-Blind Controlled Comparison of NRX-101 (D-cycloserine/ lurasidone) to Lurasidone for Adults with Bipolar Depression and Subacute Suicidal Ideation or Behavior. Am Soc Clin Psych Annual Meeting 2024.
5 Nierenberg et al. International Journal of Bipolar Disorders (2023) 11:28 https://doi.org/10.1186/s40345-023-00
6 https://www.cdc.gov/sepsis/what-is-sepsis.html
7 Open Forum Infectious Diseases, Volume 9, Issue 7, July 2022, ofac315, https://doi.org/10.1093/ofid/ofac315
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/nrx-pharmaceuticals-nasdaqnrxp-reports-second-quarter-and-year-to-date-2024-financial-results-and-provides-business-update-302222664.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/nrx-pharmaceuticals-nasdaqnrxp-reports-second-quarter-and-year-to-date-2024-financial-results-and-provides-business-update-302222664.html
SOURCE NRx Pharmaceuticals, Inc.
FAQ
What were NRx Pharmaceuticals' (NRXP) key financial results for Q2 2024?
What are NRx Pharmaceuticals' (NRXP) plans for NDA filings in 2024?
How much funding did NRx Pharmaceuticals (NRXP) secure in Q2 2024?