NLS Pharmaceutics CEO Issues Letter to Shareholders
- Completed $10 million private placement with BVF Partners L.P. to benefit narcolepsy patients
- Received non-binding term sheets for potential partnership agreement within pharmaceutical industry
- Phase 3 clinical trial for Mazindol ER in narcolepsy received IRB approval and FDA green light
- Submitted fast-track designation application for Mazindol ER to FDA
- Presented positive data on pipeline compounds at medical conferences
- None.
Insights
Analyzing...
ZURICH, SWITZERLAND / ACCESSWIRE / August 28, 2023 / NLS Pharmaceutics Ltd. (Nasdaq:NLSP, NLSPW) ("NLS" or the "Company"), a Swiss clinical-stage biopharmaceutical company focused on the discovery and development of innovative therapies for patients with rare and complex central nervous system disorders, today announced that its Chief Executive Officer, Alex Zwyer, has issued the following letter to shareholders:
NLS Pharmaceutics CEO Issues Letter to Shareholders
To our Shareholders and Friends,
Though we may have been quiet recently, I wanted to assure you that the team here at NLS has been very busy behind the scenes ensuring that we realize our vision of awakening a brighter future for patients. Your investment and support to advance our pipeline in rare sleep disorders and other rare and debilitating Central Nervous System (CNS) disorders is our driving force as we put patients first to develop better therapies to safeguard and empower the brain throughout all stages of life. I am pleased to report on the progress that we have made towards achieving both our short and our long-term goals.
At the outset of 2023, we outlined a number of objectives including:
- Continuing to build an organization dedicated to rare and complex CNS disorders
- Solidifying the opportunity for lead product Mazindol ER's success through a rigorous Phase 3 program
- Realizing Mazindol ER's full potential across a variety of rare sleep disorders
- Progressing pipeline of innovative products to meet the unmet needs of patients and transforming the lives of patients with rare diseases
In addition to making significant progress on our objectives, we have advanced in our overall efforts by participating in global medical conferences, building investor relations and partaking in discussions regarding promising opportunities. As we are at the precipice of beginning our Phase 3 program for Mazindol ER, I wanted to take the opportunity to share the promises that we have kept and the advances that we have made, which we could not have achieved without your help.
Financing
In late 2022, we completed a
We are now in the process of assessing different options to initiate our Phase 3 program as well as our strategic plan, which includes: the potential need for capital, partnerships, venture debt opportunities and business development opportunities. We have significantly reduced our monthly expenditures to extend our cash runway while finalizing our efforts. At this time we have received several non-binding term sheets for a potential partnership agreement within the pharmaceutical industry. The Company is still in negotiations, has not executed a definitive agreement, and no party is under any obligation to enter into or continue negotiations regarding a definitive agreement related to any transaction.
Leadership
In May of this year, we announced the appointment of Keith Dewedoff to the position of Interim Chief Financial Officer (CFO). Mr. Dewedoff's depth of expertise in finance within biotech, and his experience in executing growth capital initiatives, as well as corporate development and equity research, brought crucial talents into the Company at the right time. Mr. Dewedoff's contributions have been significant.
Following Mr. Dewedoff's appointment and after an exhaustive international search, in June we engaged Elena Thyen-Pighin, an experienced finance executive, to transition into the role of permanent CFO & Head of Finance / Human Resources. Ms. Thyen-Pighin's position will be effective on September 1, 2023. Ms. Thyen-Pighin holds extensive experience in leadership and management functions as both head of finance and human resources across a number of industries, including organizations similar to NLS. Based in Switzerland, Ms. Thyen-Pighin speaks 5 languages and has a strong and successful track record, most notably in accounting for both private and publicly listed enterprises. Her responsibilities will include oversight of all financial operations as well as those related to human resources. We are thrilled to welcome her to the leadership team.
Mazindol ER
In July of 2023, NLS announced that the Phase 3 clinical trial (which we call the AMAZE Program) protocol to evaluate the safety and efficacy of Mazindol ER in patients with narcolepsy type 1 received approval from the independent Institutional Review Board ("IRB"). The AMAZE Program encompasses two almost-identical double-blind Phase 3 studies (N=50 each) investigating Mazindol ER versus placebo in adult patients with narcolepsy commencing this summer at multiple sites exclusively in the U.S. Based on the U.S. Food and Drug Administration's (FDA) recommendations, both Phase 3 trials will measure the weekly cataplexy episodes as the primary endpoint over 8 weeks of treatment and excessive daytime sleepiness as a secondary objective using the Patient-Reported Outcomes Measurement Information System (PROMIS-SRI) and the Epworth Sleepiness Scale (ESS).
Along with IRB approval and the green light from the FDA, NLS has retained a contract research organization (CRO) and has enrolled a number of sites for the phase 3 studies. Once suitable capital has been secured, the phase 3 program will immediately commence as the sites are ready to begin enrolling patients.
On August 25, 2023, NLS submitted a fast-track designation application for Mazindol ER for the treatment of narcolepsy to the FDA. Fast track is a designation by the FDA of an investigational drug for expedited review to facilitate development of drugs that treat a serious or life-threatening condition and fill an unmet medical need. A drug may be granted Fast Track Designation if it is believed to have an impact on patient survival, day-to-day functioning, or if it is believed that the condition will progress in severity if left untreated. Standard reviews by the FDA for drug approval generally take about one year. A medicine that receives Fast Track Designation can be on the market within six months of the regulatory application.
Medical Congress Activities
At SLEEP 2023, the annual meeting of the American Academy of Sleep Medicine (AASM) and the Sleep Research Society (SRS), NLS presented our findings highlighting data from the recently completed Phase 2 multi-center U.S. clinical study evaluating Mazindol ER, a triple monoamine reuptake inhibitor and partial Orexin-2 Receptor agonist, in adult patients suffering from narcolepsy, which study met its primary endpoint with high statistical significance and demonstrated a favorable safety and tolerability profile. We believe that these results confirm the efficacy of Mazindol ER, as well as the safety and tolerability profile established in over 40 years of on-label and off-label use. Additional data from our Open Label Extension study (NLS-1022) further validated the positive results from our Phase 2 double-blind trial for Mazindol ER in narcolepsy and demonstrated the potential long-term efficacy, tolerability, and safety of the treatment.
In addition to presenting our findings, we spoke to hundreds of clinicians at our booth as well as presented to a packed room in our symposium entitled, Mazindol ER: Pioneering the Combination of SNDRI and OX2R in the Treatment of Narcolepsy, in which we, along with internationally renowned key opinion leaders (KOLs) in sleep medicine, Bruce Corser, M.D., Medical Director, Sleep Management Institute, Cincinnati, and Clete Kushida, MD, PhD, Chief and Medical Director, Division of Sleep Medicine, Stanford School of Medicine, presented on:
- Unmet needs in the treatment of Narcolepsy
- Phase 2 Clinical Data presentation for Mazindol ER (Studies NLS-1021 & NLS-1022)
- Phase 3 Program Summary for Mazindol ER (Studies NLS-1031, NLS-1032 & NLS-1033)
- NLS' Pipeline
Progressing our Pipeline
The NLS current pipeline bridges the present to the future, providing a holistic approach and further strengthening the Company's vision to awaken a brighter future for patients by overcoming rare and complex CNS diseases. We believe that our pipeline products are well positioned as we gain ground in establishing future market positions through patents for lauflumide (NLS-4) and other new chemical entities and assets. With extensive intellectual property (IP) coverage in the U.S., Japan and Europe, and promising pre-clinical data, we anticipate that our wake-promoting agent, lauflumide (NLS-4), could also offer a new option for the treatment of chronic fatigue, including fatigue associated with cancer treatment and long-COVID symptoms. We anticipate that clinical trials with lauflumide (NLS-4) will begin in 2024.
As we strive to develop better therapies to safeguard and empower the brain throughout all stages of life, these compounds, including Mazindol ER for the treatment of narcolepsy, along with NLS-4 focused on idiopathic hypersomnia and chronic fatigue, and NLS-11, addressing Kleine-Levin Syndrome and neurodegenerative diseases (e.g. Lewy body dementia), would offer much-needed treatment options to fill the gaps for patients with these disorders.
Current NLS pipeline products focused on Sleep disorders:
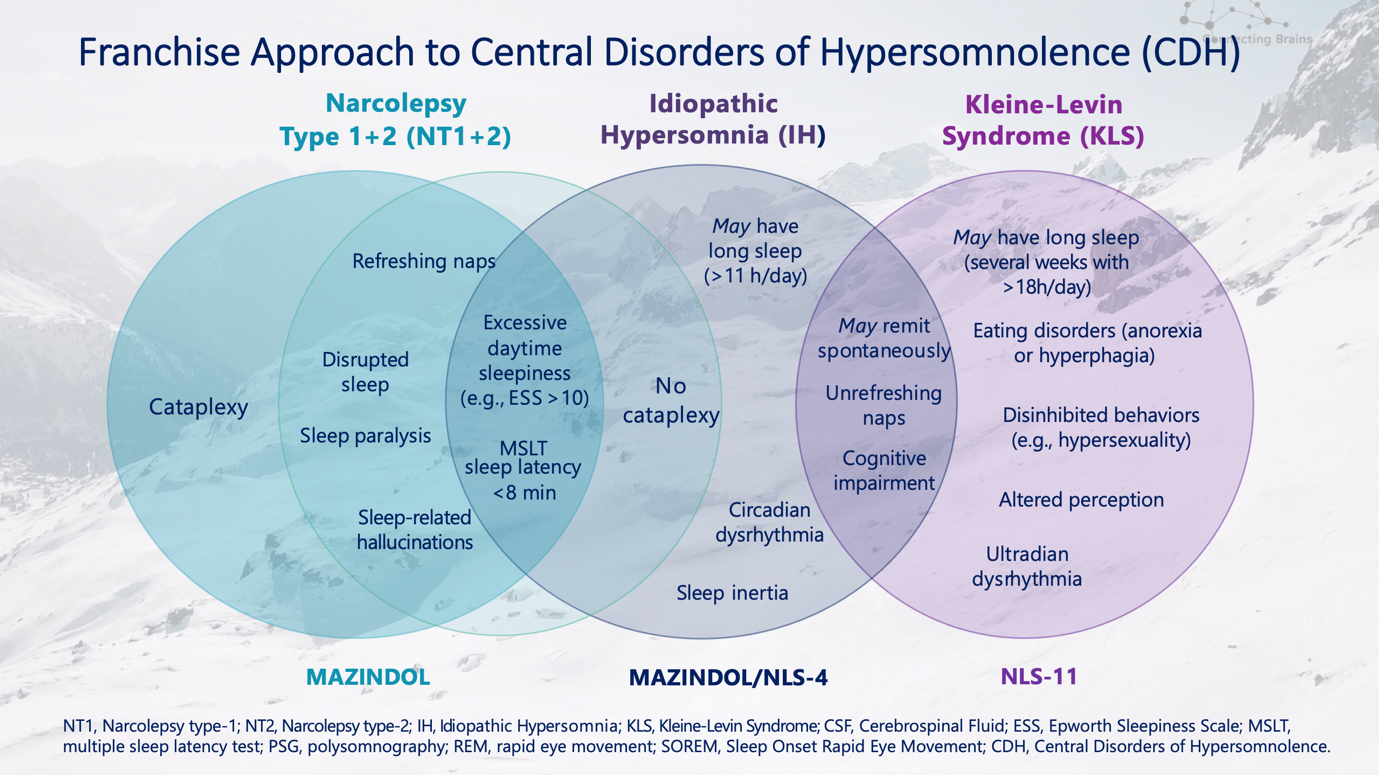
In May of this year, NLS presented new and compelling preclinical data on four of our pipeline compounds at the annual meeting of the American Society of Clinical Psychopharmacology (ASCP), in Miami, Florida. NLS' Chief Scientific Officer, Eric Konofal, MD, PhD, presented data highlighting the Company's focus on and investment in therapeutic areas of rare hypersomnia disorders and complex neurologic disorders:
- Effects of NLS-4 (Lauflumide) and modafinil in a rat model of chronic severe fatigue
- Effects of NLS-8 (Melafenoxate) on memory in a model of Alzheimer's Disease, the scopolamine-induced amnesia in the novel object recognition test in mice
- Effects of NLS-11 (Benedin) on memory in the novel object recognition test in mice
- Effects of NLS-12 (Oxafuramine) on memory in the novel object recognition test in mice
We will continue to explore opportunities and execute on possibilities based on key factors such as unmet medical needs, biological rationales, safety profiles, feasibility of clinical development, potential for leveraging accelerated development pathways for regulatory approval, strong IP positions, favorable competitive landscapes, and attractive commercial potential.
The NLS discovery platform continues to focus on single molecules that function through multiple mechanisms designed to target the complexity of the CNS disease state. Our goal remains building a differentiated global pharmaceutical company that is patient-centered and dedicated to the development of transformative therapies addressing critical unmet needs. As we navigate the competitive landscape of our industry while focusing on the development of our product candidates, we are poised to maximize the therapeutic potential of our current pipeline while still pursuing new candidates that will continue to broaden our product portfolio.
Unwavering Support
At our Annual General Meeting (AGM) in July, NLS shareholders approved all of the Board of Directors' proposals for the AGM that took place in Zürich, Switzerland on June 30, 2023. This included the election of Audrey Greenberg and Dr. Anthony Walsh to the Board of Directors, shareholder approval of financial statements, the compensation report and the balance sheet results of the Company for the fiscal year 2022. Shareholders also approved the total compensation budgets for NLS' Board of Directors and Executive Management for the financial year 2024. PricewaterhouseCoopers AG was re-elected as NLS' independent auditors for another term. Other key highlights from the meeting include
A Bright Future
More than ever, we at NLS are committed and passionate to be a part of a company with genuine near and long-term prospects to change the lives of patients with rare CNS diseases. We work together in order to create a culture that inspires and motivates our team members to do what they do best. Our leadership team remains nimble with an honest approach to transparent communication, empowerment and individual ownership of responsibilities. Our team members thrive in finding innovative and efficient approaches to advance our corporate aspirations.
Together with our co-founder, Dr. Eric Konofal, I want to offer my sincerest gratitude to the clinical investigators and participating patients making it possible for Mazindol ER to be a potential class-leading treatment for narcolepsy in the future. I must also thank the many women and men across Europe and the U.S. that are part of the NLS team, from pre-clinical to regulatory affairs to supply chain and all in between, that endeavor every day to progress our objectives in treating these rare diseases. And finally, I would like to thank you, our shareholders and investors, for your continued support for and shared commitment to NLS.
With gratitude,
Alex Zwyer
Chief Executive Officer
Safe Harbor Statement
This press release contains expressed or implied forward-looking statements pursuant to U.S. Federal securities laws. For example, NLS is using forward-looking statements when it discusses the potential benefits to be derived from its product candidates, the indications that its product candidates may address, its potential partnership opportunities from the receipt of several non-binding term sheets, the timing of the commencement of its studies, the potential for Mazindol ER to receive fast-track designation by the FDA, its strategic plan, the potential need for capital, business development opportunities, and its belief that its pipeline products are well positioned as it gains ground in establishing future market positions with its other assets. These forward-looking statements and their implications are based on the current expectations of the management of NLS only and are subject to a number of factors and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements. The following factors, among others, could cause actual results to differ materially from those described in the forward-looking statements: changes in technology and market requirements; NLS may encounter delays or obstacles in launching and/or successfully completing its clinical trials; NLS' products may not be approved by regulatory agencies, NLS' technology may not be validated as it progresses further and its methods may not be accepted by the scientific community; NLS may be unable to retain or attract key employees whose knowledge is essential to the development of its products; unforeseen scientific difficulties may develop with NLS' process; NLS' products may wind up being more expensive than it anticipates; results in the laboratory may not translate to equally good results in real clinical settings; results of preclinical studies may not correlate with the results of human clinical trials; NLS' patents may not be sufficient; NLS' products may harm recipients; changes in legislation may adversely impact NLS; inability to timely develop and introduce new technologies, products and applications; and loss of market share and pressure on pricing resulting from competition, which could cause the actual results or performance of NLS to differ materially from those contemplated in such forward-looking statements. Except as otherwise required by law, NLS undertakes no obligation to publicly release any revisions to these forward-looking statements to reflect events or circumstances after the date hereof or to reflect the occurrence of unanticipated events. More detailed information about the risks and uncertainties affecting NLS is contained under the heading "Risk Factors" in NLS' annual report on Form 20-F for the year ended December 31, 2022 filed with the Securities and Exchange Commission (SEC), which is available on the SEC's website, www.sec.gov, and in subsequent filings made by NLS with the SEC.
For additional information:
Marianne Lambertson (investors & media)
NLS Pharmaceutics Ltd.
+1 239.682.8500
ml@nls-pharma.com
www.nlspharmaceutics.com
###
SOURCE: NLS Pharmaceutics AG
View source version on accesswire.com:
https://www.accesswire.com/777605/NLS-Pharmaceutics-CEO-Issues-Letter-to-Shareholders







