Endo Expands Voluntary Recall of Clonazepam Orally Disintegrating Tablets, USP (C-IV) Due to Potential Product Carton Strength Mislabeling
Rhea-AI Summary
Endo USA (OTCQX: NDOI) is expanding its voluntary recall of Clonazepam Orally Disintegrating Tablets due to potential carton strength mislabeling caused by a third-party packager error. The recall affects multiple lots of various strengths (0.125mg, 0.25mg, 1mg, and 2mg). While blister strips and tablets contain correct strengths, cartons may display incorrect strength and NDC codes. This mislabeling poses risks of adverse effects including sedation, confusion, and potentially life-threatening respiratory depression. No adverse events have been reported. The company is notifying distributors and retailers to stop distribution and return existing inventory through Inmar, Inc.
Positive
- No adverse events reported related to the recall
Negative
- Expanded product recall affecting multiple product lots and strengths
- Risk of serious adverse effects including life-threatening respiratory depression
- Potential business disruption and inventory returns from distributors and retailers
- Possible reputational damage due to packaging quality control issues
News Market Reaction – NDOI
On the day this news was published, NDOI gained 0.20%, reflecting a mild positive market reaction.
Data tracked by StockTitan Argus on the day of publication.
Specifically, Endo's ongoing investigation has identified the possibility that the Clonazepam product lots listed below contain a limited number of cartons printed with the incorrect strength and National Drug Code (NDC) code due to an error by a third-party packager. The blister strips and tablets inside the product pack reflect the correct strength for the lot.
The following table details the lots being added to the voluntary recall, including lot product description and NDC number.
Potential Product Description / NDC Number | Lot # |
Clonazepam ODT, USP (C-IV) 2mg / 49884-310-02 | 550176501 |
550176601 | |
Clonazepam ODT, USP (C-IV) 0.125mg / 49884-306-02 | 550174101 |
Clonazepam ODT, USP (C-IV) 0.25mg / 49884-307-02 | 550142801 |
550142901 | |
550143001 | |
550143101 | |
550143201 | |
550143301 | |
550143401 | |
550147201 | |
550147401 | |
Clonazepam ODT, USP (C-IV) 1mg / 49884-309-02 | 550145201 |
550175901 | |
550176001 | |
550176201 |
Risk Statement:
Children and adults who inadvertently consume a higher dose of clonazepam could be at increased risk for the adverse events of significant sedation, confusion, dizziness, diminished reflexes, ataxia, and hypotonia. There is reasonable probability for significant, possibly life-threatening, respiratory depression especially for patients with concomitant pulmonary disease, patients who have prescribed dosing near maximal dosing, and patients also taking other medications that could cause additional respiratory depression.
To date, Endo has not received any reports of adverse events associated with this product recall.
Clonazepam Orally Disintegrating Tablets are indicated alone or as an adjunct in the treatment of the Lennoz-Gastaut syndrome (petit mal variant), akinetic and myoclonic seizures. Additionally, the product is indicated for the treatment of panic disorder.
Package Identification:
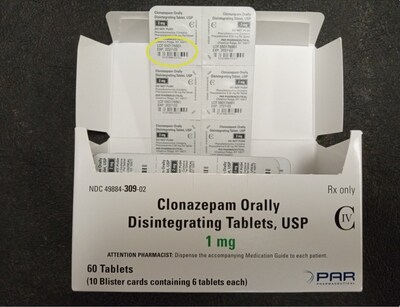
The product is packaged in cartons containing 60 tablets packed into 10 blister strips each containing 6 tablets. The carton and each blister strip pocket are printed with the name, strength, lot number, expiration date, and NDC number. The packaging lists the legacy company Par Pharmaceutical which previously marketed clonazepam before the product was acquired by Endo.
The images below provide an example of the potential mislabeling showing the components of a package of Clonazepam Orally Disintegrating tablets, USP 2 mg lot 550176501 with a carton bearing the product description and NDC code of Clonazepam Orally Disintegrating Tablets, USP 1 mg 60-count. The location of the lot number on each component of the package is shown on the photographs within this release.
Action Required:
The product lots were distributed through wholesale distributors to retail pharmacies nationwide.
Endo is providing written notification to wholesale accounts and retailers that have received the product lots and is arranging for the return of all existing inventory through Inmar, Inc.
Distributors, retailers that have the product lot being recalled should immediately stop distributing and dispensing and return to the place of purchase or contact Inmar on the below telephone line.
Consumers in possession of any unused prescribed tablet cartons of Clonazepam Orally Disintegrating tablets, USP bearing the above lot numbers have been advised to discontinue use of the product.
In the event that a patient inadvertently took an incorrect dose rather than the intended dose, they are advised to consult a physician.
Consumers with questions regarding this recall can contact Inmar by telephone at 855-589- 1869 (Monday through Friday, 9 a.m. to 5 p.m. ET) or by email at rxrecalls@inmar.com.
For more information about Clonazepam Orally Disintegrating Tablets, USP, please see full Prescribing Information including BOXED WARNING available at DailyMed - CLONAZEPAM tablet, orally disintegrating (nih.gov).
Adverse reactions or quality problems experienced with the use of this product lot may be reported to the U.S. Food and Drug Administration's (FDA) MedWatch Adverse Event Reporting program:
- Online: www.fda.gov/medwatch/report.htm
- Mail: use postage-paid, pre-addressed Form FDA 3500 available at www.fda.gov/MedWatch/getforms.htm
- Fax: 1-800-FDA-0178
This product lot recall is being made with the FDA's knowledge.
About Endo
Endo is a diversified pharmaceutical company boldly transforming insights into life- enhancing therapies. Our passionate team members collaborate to develop and deliver these essential medicines. Together, we are committed to helping everyone we serve live their best life. Learn more at www.endo.com or connect with us on LinkedIn.
Cautionary Note Regarding Forward-Looking Statements
This press release contains forward-looking statements including but not limited to any statements related to product recalls, mislabeling, adverse events, FDA or other regulatory actions and any other statements that refer to expected, estimated or anticipated future results or that do not relate solely to historical facts. Statements including words such as "believes," "expects," "anticipates," "intends," "estimates," "plan," "will," "may," "look forward," "guidance," "future," "potential" or similar expressions are forward-looking statements. Because these statements reflect Endo's current views, expectations and beliefs concerning future events, they involve risks and uncertainties, some of which Endo may not currently be able to predict. Although Endo believes that these forward-looking statements and other information are based upon reasonable assumptions and expectations, readers should not place undue reliance on these or any other forward-looking statements and information. Actual results may differ materially and adversely from current expectations based on a number of risks, uncertainties and factors, including risks and uncertainties related to the recall and any future recalls, potential adverse events and any regulatory actions by the FDA. Endo assumes no obligation to publicly update any forward- looking statements, whether as a result of new information, future developments or otherwise, except as may be required under applicable securities laws. Additional information concerning risk factors, including those referenced above, can be found in press releases issued by Endo and in Endo's public filings with the
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/endo-expands-voluntary-recall-of-clonazepam-orally-disintegrating-tablets-usp-c-iv-due-to-potential-product-carton-strength-mislabeling-302309074.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/endo-expands-voluntary-recall-of-clonazepam-orally-disintegrating-tablets-usp-c-iv-due-to-potential-product-carton-strength-mislabeling-302309074.html
SOURCE Endo, Inc.