Neurocrine Biosciences Announces Publication of Primary CAHtalyst™ Adult Phase 3 Study Results of Crinecerfont for the Treatment of CAH in The New England Journal of Medicine
Neurocrine Biosciences announced that the CAHtalyst™ Phase 3 study results of crinecerfont for treating adults with congenital adrenal hyperplasia (CAH) were published in the New England Journal of Medicine. The study met its primary and key secondary endpoints, showing significant reductions in glucocorticoid (GC) doses while maintaining androgen control. 62.7% of crinecerfont-treated participants achieved a physiologic GC dose versus 17.5% with placebo. Crinecerfont was generally well tolerated, with the most common adverse events being fatigue and headache. The study involved 182 participants, with over 95% completing the 24-week double-blind period.
Crinecerfont reduced androstenedione levels more than placebo, demonstrating its efficacy through a GC-independent mechanism. Participants saw improvements in metrics related to long-term GC therapy such as body weight and glucose tolerance. The treatment showed no major safety concerns, with few serious adverse events reported.
- Primary and key secondary endpoints met, showing efficacy in reducing GC doses while maintaining androgen control.
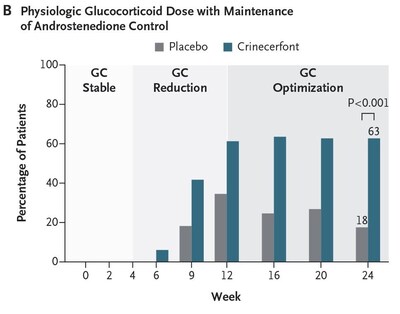
- 62.7% of crinecerfont-treated participants achieved a physiologic GC dose versus 17.5% with placebo.
- Significant reduction in androstenedione levels with crinecerfont compared to placebo.
- Favorable trends in endpoints associated with long-term GC therapy, such as body weight and glucose tolerance.
- Crinecerfont was generally well tolerated; most common adverse events were fatigue and headache.
- Over 95% of participants completed the 24-week double-blind period.
- Few serious adverse events reported, none assessed as related to crinecerfont.
- Despite favorable trends, the short-term study duration may not fully capture long-term safety and efficacy.
- Adverse events, although mild, included fatigue and headache which could impact patient compliance.
Insights
Neurocrine Biosciences' publication of the CAHtalyst™ Adult Phase 3 study results in The New England Journal of Medicine is a significant milestone. The study results indicate that crinecerfont effectively reduces androstenedione levels and allows for the reduction of glucocorticoid (GC) doses while maintaining androgen control. This treatment addresses a long-standing issue of excessive androgen production in congenital adrenal hyperplasia (CAH) patients, which has traditionally led to various health conditions due to the necessity of high GC doses.
From a medical perspective, the ability of crinecerfont to achieve these outcomes through a non-GC mechanism is a substantial breakthrough. This will likely shift the therapeutic paradigm for CAH patients, reducing the risk of comorbidities such as osteoporosis, obesity and diabetes, which are common with long-term high-dose GC therapy. The safety profile of crinecerfont, with minimal serious adverse events, further underlines its potential as a preferred treatment option.
For investors, the publication in such a prestigious journal validates the robustness of the study and enhances the credibility of Neurocrine Biosciences' research efforts. This can be expected to positively influence the company's stock, as it reflects both scientific and therapeutic advancements.
The publication of Phase 3 study results for crinecerfont in a high-impact journal is a significant event for Neurocrine Biosciences. The achievement of primary and key secondary endpoints, with 62.7% of participants achieving a physiologic GC dose compared to 17.5% on placebo, underscores the potential market impact and commercial viability of crinecerfont.
From a financial perspective, the positive clinical data supports the potential for regulatory approval, which could open up a new revenue stream for the company. The CAH treatment market is substantial, given that CAH is a chronic condition requiring long-term management. The ability of crinecerfont to address both efficacy and safety concerns positions it as a competitive product in this market.
Investors should note the favorable tolerability profile and the potential for crinecerfont to mitigate the side effects associated with current CAH treatments. This could lead to strong market adoption and a significant uptick in company revenues post-approval. The publication also serves to boost investor confidence in the company's pipeline and overall research and development capabilities.
The successful results from the CAHtalyst™ Adult Phase 3 study and their publication in The New England Journal of Medicine mark a pivotal moment for Neurocrine Biosciences. Crinecerfont's ability to significantly reduce GC doses while controlling androgen levels addresses a critical unmet need in the CAH market. This should enhance the drug's market positioning and provide a competitive edge over existing treatments.
Market dynamics for CAH treatments are likely to shift in favor of crinecerfont, given its efficacy and safety profile. The data suggests a strong potential for physician adoption, especially for patients struggling with the adverse effects of long-term high GC doses. Additionally, the favorable trends observed in terms of body weight, insulin resistance and glucose tolerance after short-term GC dose reduction are promising for long-term health outcomes, which will likely appeal to both patients and healthcare providers.
For retail investors, the breakthrough nature of these results, coupled with the strong safety profile, suggests a robust market entry for crinecerfont. The drug’s potential to become a standard treatment for CAH could drive significant long-term revenue growth for Neurocrine Biosciences, enhancing shareholder value.
- CAHtalyst™ Adult Phase 3 Study Met Primary and Important Key Secondary Endpoints, with Crinecerfont Treatment Decreasing Androstenedione Levels and Enabling Glucocorticoid Dose Reduction While Maintaining Androstenedione Control
-
- Favorable Trends in Endpoints Reflecting Consequences of Supraphysiologic Glucocorticoid Dosing
- Crinecerfont was Generally Well Tolerated
"Since the 1950s, glucocorticoids have been required not only for cortisol replacement, but also to control the excessive amount of adrenal androgens in patients with congenital adrenal hyperplasia. As a result, CAH patients suffer from a higher prevalence of disorders attributable to supraphysiologic GC levels, including osteoporosis, obesity, insulin resistance, diabetes mellitus, hyperlipidemia and hypertension," said Dr. Richard Auchus, M.D., Ph.D., Principal Investigator, Professor of Pharmacology and Internal Medicine, Division of Metabolism, Endocrinology, and Diabetes at the University of
Crinecerfont is an investigational, oral, selective corticotropin-releasing factor type 1 receptor (CRF1) antagonist being developed to reduce and control excess adrenocorticotropic hormone (ACTH) and adrenal androgens through a GC-independent mechanism for the treatment of CAH.
"The CAHtalyst Adult Phase 3 study demonstrated the ability of crinecerfont to substantially reduce glucocorticoid doses to more physiologic levels while maintaining or improving androgen control," said Eiry W. Roberts, M.D., Chief Medical Officer at Neurocrine Biosciences. "This represents a potential paradigm shift in treatment for adult patients living with this disorder over many years, who consistently struggle with the clinical consequences of poor androgen control and/or excess glucocorticoid caused by current inadequate treatment approaches."
The CAHtalyst Adult Phase 3 global registrational study was conducted in 182 participants and designed to evaluate the safety, efficacy, and tolerability of crinecerfont in adults ages 18 years and older with CAH due to 21-hydroxylase deficiency. Over
The Phase 3 Adult study met the primary endpoint of percent change from baseline in GC dose (while maintaining androstenedione control) and the key secondary endpoint of achievement of reduction to a physiologic GC dose (while maintaining androstenedione control) at Week 24.
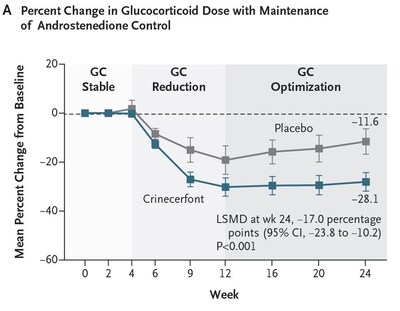
- Crinecerfont treatment led to a significantly greater GC dose reduction at Week 24 while maintaining androstenedione control compared to placebo (least squares mean [LSM] percent change from baseline of -
27.3% versus -10.3% , with LS mean difference [LSMD] of -17.0% , P < 0.001). Actual value is P < 0.0001. (Figure A)- This corresponded to changes of -4.8 and -2.1 mg/m2/day hydrocortisone equivalents for crinecerfont and placebo, respectively
- After the initial 4-week GC stable period, mean percent GC reduction (while androstenedione was controlled) was greater with crinecerfont than placebo at all timepoints, and this effect was maintained from Weeks 12 to 24, while GC levels increased from Weeks 12 to 24 for participants on placebo.
Importantly, a significantly greater proportion of participants treated with crinecerfont achieved reduction to a physiologic GC dose (≤ 11 mg/m2/day) while maintaining androstenedione control compared to placebo (
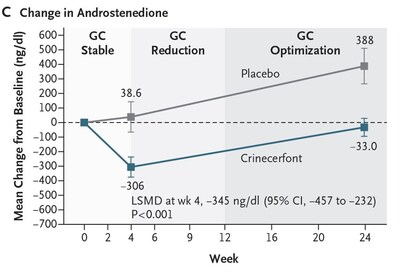
The Phase 3 Adult study also met the key secondary endpoint of change from baseline in androstenedione (key adrenal androgen) following the initial 4-week GC stable period:
- Crinecerfont treatment led to a significantly greater reduction in androstenedione compared to an increase with placebo at Week 4 (LS mean change from baseline of -299 ng/dL versus +45.5 ng/dL, with LSMD of -345 ng/dL; P < 0.001). Actual value is P < 0.0001.
- The significant reduction in androstenedione levels while GC was stable provides clear evidence of the ability of crinecerfont to reduce elevated adrenal androgen levels through a non-GC mechanism.
At Week 24, mean androstenedione levels remained below baseline with crinecerfont in the context of substantially reduced GC dose. However, androstenedione levels increased to above baseline with placebo, highlighting the inability to maintain androgen control when GC was reduced in the absence of any other intervention. (Figure C)
Despite the short-term duration of this Phase 3 study, favorable trends were observed for endpoints associated with long-term supraphysiologic GC therapy such as body weight, insulin resistance, and glucose tolerance that were encouraging considering the relatively short time frame of reduced GC dose. In addition, bone resorption and formation markers increased more with crinecerfont, reflecting relief from GC-induced suppression of bone turnover.
Crinecerfont was generally well tolerated, with the most common adverse events being fatigue and headache. Few serious adverse events occurred, with none assessed as related to crinecerfont, and there were few discontinuations due to adverse events. There was no safety concern related to adrenal crisis, with few events reported during the double-blind period and similar incidence of adverse events leading to glucocorticoid stress dosing in the crinecerfont and placebo groups.
Note: P-values in the text and figures reflect the Journal's convention of calculating to two significant figures. Actual P-values to three significant figures are also provided. Figures reflect observed means rather than the LSM and LSMD described in the text.
Figures are from The New England Journal of Medicine, Phase 3 Trial of Crinecerfont in Adult Congenital Adrenal Hyperplasia, Auchus R, Hamidi O, Pivonello R, et.al. This article was published online on June 1, 2024. Copyright © 2024 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
About Congenital Adrenal Hyperplasia
Congenital adrenal hyperplasia (CAH) is a rare genetic condition that results in an enzyme deficiency that alters the production of adrenal hormones which are essential for life. Approximately
Glucocorticoids (GCs) are currently used not only to correct the endogenous cortisol deficiency, but doses used are higher than cortisol replacement needed (supraphysiologic) to lower the levels of adrenocorticotropic hormone (ACTH) and adrenal androgens. However, glucocorticoid treatment at supraphysiologic doses has been associated with serious and significant complications of steroid excess, including metabolic issues such as weight gain and diabetes, cardiovascular disease, and osteoporosis. Additionally, long-term treatment with supraphysiologic GC doses may have psychological and cognitive impact, such as changes in mood and memory. Adrenal androgen excess has been associated with abnormal bone growth and development in pediatric patients, female health problems such as acne, excess hair growth and menstrual irregularities, testicular rest tumors in males, and fertility issues in both sexes.
To learn more about CAH, click here.
About Crinecerfont and the CAHtalyst™ Studies
Crinecerfont is an investigational, oral, selective corticotropin-releasing factor type 1 receptor (CRF1) antagonist being developed to reduce and control excess adrenocorticotropic hormone (ACTH) and adrenal androgens through a glucocorticoid-independent mechanism for the treatment of congenital adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency. Antagonism of CRF1 receptors in the pituitary has been shown to decrease ACTH levels, which in turn decreases the production of adrenal androgens and potentially the symptoms associated with CAH. Our data demonstrate that lowering adrenal androgen levels enables lower, more physiologic dosing of glucocorticoids and thus could potentially reduce the complications associated with exposure to greater than normal glucocorticoid doses in patients with CAH.
The CAHtalyst™ Pediatric and Adult Phase 3 global registrational studies are designed to evaluate the safety, efficacy, and tolerability of crinecerfont in children and adolescents, and adults respectively, with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. The primary portions of the CAHtalyst Phase 3 studies have completed and enrollment is closed, while the open-label treatment portions of both studies are ongoing. Data from the CAHtalyst Pediatric and CAHtalyst Adult Phase 3 studies supported two New Drug Application submissions to the
To learn more about crinecerfont and the CAHtalyst studies, click here.
About Neurocrine Biosciences, Inc.
Neurocrine Biosciences is a leading neuroscience-focused, biopharmaceutical company with a simple purpose: to relieve suffering for people with great needs, but few options. We are dedicated to discovering and developing life-changing treatments for patients with under-addressed neurological, neuroendocrine and neuropsychiatric disorders. The company's diverse portfolio includes FDA-approved treatments for tardive dyskinesia, chorea associated with Huntington's disease, endometriosis* and uterine fibroids*, as well as a robust pipeline including multiple compounds in mid- to late-phase clinical development across our core therapeutic areas. For three decades, we have applied our unique insight into neuroscience and the interconnections between brain and body systems to treat complex conditions. We relentlessly pursue medicines to ease the burden of debilitating diseases and disorders, because you deserve brave science. For more information, visit neurocrine.com, and follow the company on LinkedIn, X (formerly Twitter), and Facebook.
(*in collaboration with AbbVie)
The NEUROCRINE BIOSCIENCES Logo Lockup and YOU DESERVE BRAVE SCIENCE are registered trademarks of Neurocrine Biosciences, Inc. CAHtalyst is a trademark of Neurocrine Biosciences, Inc.
Forward-Looking Statements
In addition to historical facts, this press release contains forward-looking statements that involve a number of risks and uncertainties. These statements include, but are not limited to, statements regarding the potential benefits to be derived from crinecerfont. Among the factors that could cause actual results to differ materially from those indicated in the forward-looking statements include: the crinecerfont NDAs may not be accepted for filing by the FDA or may not obtain regulatory approval or such approval may be delayed; additional regulatory submissions may not occur or be submitted in a timely manner; the FDA may make adverse decisions regarding crinecerfont; crinecerfont may not be found to be safe and/or effective or may not prove to be beneficial to patients; development activities for crinecerfont may not be completed on time or at all; clinical development activities may be delayed for regulatory or other reasons, may not be successful or replicate previous and/or interim clinical trial results, or may not be predictive of real-world results or of results in subsequent clinical trials; competitive products and technological changes that may limit demand for our products; uncertainties relating to patent protection and intellectual property rights of third parties; our dependence on third parties for development and manufacturing activities related to crinecerfont, and our ability to manage these third parties; our future financial and operating performance; risks and uncertainties associated with the commercialization of our products; and other risks described in the Company's periodic reports filed with the Securities and Exchange Commission, including without limitation the Company's quarterly report on Form 10-Q for the quarter ended March 31, 2024. Neurocrine Biosciences disclaims any obligation to update the statements contained in this press release after the date hereof.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/neurocrine-biosciences-announces-publication-of-primary-cahtalyst-adult-phase-3-study-results-of-crinecerfont-for-the-treatment-of-cah-in-the-new-england-journal-of-medicine-302161424.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/neurocrine-biosciences-announces-publication-of-primary-cahtalyst-adult-phase-3-study-results-of-crinecerfont-for-the-treatment-of-cah-in-the-new-england-journal-of-medicine-302161424.html
SOURCE Neurocrine Biosciences, Inc.