Neurocrine Biosciences Announces Publication of Primary CAHtalyst™ Pediatric Phase 3 Study Results of Crinecerfont for the Treatment of CAH in The New England Journal of Medicine
Neurocrine Biosciences announced the publication of its CAHtalyst Pediatric Phase 3 study results in The New England Journal of Medicine, showcasing crinecerfont's efficacy in treating congenital adrenal hyperplasia (CAH). The study met both primary and key secondary endpoints, with crinecerfont successfully lowering androstenedione levels and allowing glucocorticoid dose reduction while maintaining androgen control. Notably, 30% of participants on crinecerfont achieved a physiologic glucocorticoid dose at 28 weeks, compared to 0% in the placebo group. The treatment also exhibited favorable trends in mitigating long-term effects of high-dose glucocorticoid and androgen excess, with improvements in body weight, insulin resistance, and hirsutism. Crinecerfont was generally well tolerated, with common adverse events including headache, fever, and vomiting.
- CAHtalyst Pediatric Phase 3 study met primary and key secondary endpoints.
- Crinecerfont significantly reduced androstenedione levels compared to placebo.
- 30% of Crinecerfont participants achieved a physiologic glucocorticoid dose at Week 28.
- Favorable trends in body weight, insulin resistance, and reduced hirsutism.
- Crinecerfont was generally well tolerated with minimal serious adverse events.
- Over 95% of participants completed the 28-week double-blind period.
- Despite the successful outcomes, the study is relatively short-term, raising questions about long-term efficacy and safety.
- Adverse events like headache, fever, and vomiting were reported.
- Minimal serious adverse events were noted, but long-term safety remains uncertain.
Insights
Neurocrine Biosciences has achieved significant milestones with its CAHtalyst™ Pediatric Phase 3 study for crinecerfont, positioning it as a noteworthy advancement in treating congenital adrenal hyperplasia (CAH). The study results, published in The New England Journal of Medicine, enhance the credibility and visibility of Neurocrine's research efforts, potentially boosting investor confidence.
One standout metric is the 30% of participants achieving a physiologic glucocorticoid (GC) dose while maintaining androgen control—compared to 0% in the placebo group. These results suggest crinecerfont could significantly reduce dependency on high-dose GC treatments, which are known to have adverse long-term effects. This could translate into strong market potential and, if approved by regulatory bodies, a substantial increase in market share.
Financially, Neurocrine's successful Phase 3 trial results could lead to accelerated approval pathways, increasing the company's valuation. The positive results may drive up the company's stock price as investors anticipate future revenues from crinecerfont sales. However, it is essential to monitor regulatory reviews and potential competition in this therapeutic niche.
The CAHtalyst™ Pediatric Phase 3 study results for crinecerfont represent a promising advancement for the treatment of congenital adrenal hyperplasia (CAH). The primary and secondary endpoints—androstenedione reduction and GC dose reduction while maintaining androgen control—were met with statistical significance, highlighting the potential efficacy of crinecerfont.
Of particular interest is the mechanism of action of crinecerfont as a selective corticotropin-releasing factor type 1 receptor (CRF1) antagonist. This non-glucocorticoid approach to managing CAH addresses a significant unmet need by potentially mitigating the adverse effects associated with long-term high-dose GC therapy. The favorable outcomes in reducing body weight, improving insulin resistance and lowering hirsutism further enhance the therapeutic profile of crinecerfont.
Given these results, the medical community may view crinecerfont as a transformative treatment option, pending further validation from regulatory agencies. The publication in The New England Journal of Medicine underscores the study's scientific rigor and enhances its acceptance among healthcare professionals.
- CAHtalyst™ Pediatric Study Met Primary and Key Secondary Endpoints, with Crinecerfont Treatment Decreasing Androstenedione Levels and Enabling Glucocorticoid Dose Reduction While Maintaining Androstenedione Control
-
- Favorable Trends in Endpoints Reflecting Supraphysiologic Glucocorticoid Dosing and Androgen Excess
- Crinecerfont was Generally Well Tolerated
"In treating children with CAH, from the moment they are diagnosed we are challenged with finding a balance between supraphysiologic glucocorticoid dosing and the need to prevent androgen excess. Tipping the scale too far one way or the other can adversely impact growth and pubertal development," said Kyriakie Sarafoglou, M.D., Professor, Department of Pediatrics and Department of Experimental and Clinical Pharmacology, Divisions of Endocrinology and Genetics & Metabolism, at the University of
Crinecerfont is an investigational, oral, selective corticotropin-releasing factor type 1 receptor (CRF1) antagonist being developed to reduce and control excess adrenocorticotropic hormone (ACTH) and adrenal androgens through a GC-independent mechanism for the treatment of CAH.
"The outstanding results achieved in the CAHtalyst Pediatric Phase 3 study demonstrate the ability of crinecerfont to reduce elevated androstenedione levels while also enabling reduction in supraphysiologic glucocorticoid doses to more physiologic level dosing while maintaining androgen control in pediatric participants with CAH," said Eiry W. Roberts, M.D., Chief Medical Officer at Neurocrine Biosciences. "This could potentially enable physicians to fundamentally change their approach to the important task of controlling androgens in pediatric patients with crinecerfont, without the need for long-term supraphysiologic steroid dosing, thus removing the burden that long-term excess glucocorticoid use places on development and outcome for these patients."
The CAHtalyst Pediatric Phase 3 global registrational study was conducted in 103 participants and designed to evaluate the safety, efficacy, and tolerability of crinecerfont in children and adolescents ages 2 to 17 years with CAH due to 21-hydroxylase deficiency. Over
The Phase 3 Pediatric study met the primary endpoint of change from baseline in androstenedione (key adrenal androgen) following the initial 4-week GC-stable period.
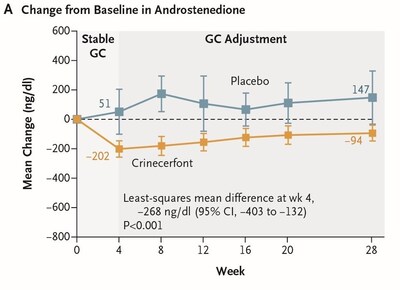
- Crinecerfont treatment led to a significantly greater reduction in androstenedione compared to an increase with placebo at Week 4 (least squares mean [LSM] change from baseline of -196.8 ng/dL versus +71.0 ng/dL, with least squares mean difference [LSMD] of -268 ng/dL, P < 0.001). Actual value is P < 0.0001. (Figure A)
After Week 4 and through Week 28, mean androstenedione remained below baseline in the context of reduced GC dose with crinecerfont but increased above baseline in the placebo group despite increased GC dose, highlighting the challenge of maintaining androgen control even with supraphysiologic GC doses in pediatric patients enrolled in this trial.
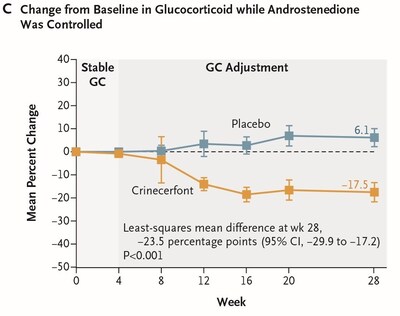
The Phase 3 Pediatric study also met the key secondary endpoint of percent change from baseline in GC dose (while maintaining androstenedione control) and the secondary endpoint of achievement of reduction to a physiologic GC dose (while maintaining androstenedione control) at Week 28:
- Crinecerfont treatment led to a significantly greater GC dose reduction at Week 28 while maintaining androstenedione control compared to placebo (LSM change from baseline of -
18% versus +5.6% , with LSMD of -23.5% ; P < 0.001). Actual value is P < 0.0001. (Figure C)
- Importantly,
30% of crinecerfont-treated participants achieved a physiologic GC dose (≤ 11 mg/m2/day hydrocortisone equivalents) at Week 28 while maintaining androstenedione control, compared to zero participants in the placebo group (nominal P-value = 0.0009). (Figure D)
Despite the short-term duration of this Phase 3 study, favorable trends were observed for endpoints associated with long-term supraphysiologic GC therapy such as body mass index standard deviation score and insulin resistance that were encouraging considering the relatively short time frame of reduced GC dose. In addition, there was reduction in hirsutism (excessive hair growth) in female participants and improvement in the androstenedione to testosterone ratio in male participants.
Crinecerfont was generally well tolerated, with the most common adverse events being headache, fever and vomiting. There were no adrenal crises in either group. Few serious adverse events occurred, with none assessed as related to crinecerfont, and there were few discontinuations due to adverse events. No events of adrenal crisis were reported during the double-blind period, and the incidence of adverse events leading to glucocorticoid stress dosing was similar in the crinecerfont and placebo groups.
Note: P-values in the text and figures reflect the Journal's convention of calculating to two significant figures. Actual P-values to three significant figures are also provided. Figures reflect observed means rather than the LSM and LSMD described in the text.
Figures are from The New England Journal of Medicine, Phase 3 Trial of Crinecerfont in Pediatric Congenital Adrenal Hyperplasia, Sarafoglou K, Kim M, Lodish M, et.al. This article was published online on June 2, 2024. Copyright © 2024 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
About Congenital Adrenal Hyperplasia
Congenital adrenal hyperplasia (CAH) is a rare genetic condition that results in an enzyme deficiency that alters the production of adrenal hormones which are essential for life. Approximately
Glucocorticoids (GCs) are currently used not only to correct the endogenous cortisol deficiency, but doses used are higher than cortisol replacement needed (supraphysiologic) to lower the levels of adrenocorticotropic hormone (ACTH) and adrenal androgens. However, glucocorticoid treatment at supraphysiologic doses has been associated with serious and significant complications of steroid excess, including metabolic issues such as weight gain and diabetes, cardiovascular disease, and osteoporosis. Additionally, long-term treatment with supraphysiologic GC doses may have psychological and cognitive impact, such as changes in mood and memory. Adrenal androgen excess has been associated with abnormal bone growth and development in pediatric patients, female health problems such as acne, excess hair growth and menstrual irregularities, testicular rest tumors in males, and fertility issues in both sexes.
To learn more about CAH, click here.
About Crinecerfont and the CAHtalyst™ Studies
Crinecerfont is an investigational, oral, selective corticotropin-releasing factor type 1 receptor (CRF1) antagonist being developed to reduce and control excess adrenocorticotropic hormone (ACTH) and adrenal androgens through a glucocorticoid-independent mechanism for the treatment of congenital adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency. Antagonism of CRF1 receptors in the pituitary has been shown to decrease ACTH levels, which in turn decreases the production of adrenal androgens and potentially the symptoms associated with CAH. Our data demonstrate that lowering adrenal androgen levels enables lower, more physiologic dosing of glucocorticoids and thus could potentially reduce the complications associated with exposure to greater than normal glucocorticoid doses in patients with CAH.
The CAHtalyst™ Pediatric and Adult Phase 3 global registrational studies are designed to evaluate the safety, efficacy, and tolerability of crinecerfont in children and adolescents, and adults respectively, with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. The primary portions of the CAHtalyst Phase 3 studies have completed and enrollment is closed, while the open-label extension treatment portions of both studies are ongoing. Data from the CAHtalyst Pediatric and CAHtalyst Adult Phase 3 studies supported two New Drug Application submissions to the
To learn more about crinecerfont and the CAHtalyst studies, click here.
About Neurocrine Biosciences, Inc.
Neurocrine Biosciences is a leading neuroscience-focused, biopharmaceutical company with a simple purpose: to relieve suffering for people with great needs, but few options. We are dedicated to discovering and developing life-changing treatments for patients with under-addressed neurological, neuroendocrine and neuropsychiatric disorders. The company's diverse portfolio includes FDA-approved treatments for tardive dyskinesia, chorea associated with Huntington's disease, endometriosis* and uterine fibroids*, as well as a robust pipeline including multiple compounds in mid- to late-phase clinical development across our core therapeutic areas. For three decades, we have applied our unique insight into neuroscience and the interconnections between brain and body systems to treat complex conditions. We relentlessly pursue medicines to ease the burden of debilitating diseases and disorders, because you deserve brave science. For more information, visit neurocrine.com, and follow the company on LinkedIn, X (formerly Twitter), and Facebook.
(*in collaboration with AbbVie)
The NEUROCRINE BIOSCIENCES Logo Lockup and YOU DESERVE BRAVE SCIENCE are registered trademarks of Neurocrine Biosciences, Inc. CAHtalyst is a trademark of Neurocrine Biosciences, Inc.
Forward-Looking Statements
In addition to historical facts, this press release contains forward-looking statements that involve a number of risks and uncertainties. These statements include, but are not limited to, statements regarding the potential benefits to be derived from crinecerfont. Among the factors that could cause actual results to differ materially from those indicated in the forward-looking statements include: the crinecerfont NDAs may not be accepted for filing by the FDA or may not obtain regulatory approval or such approval may be delayed; additional regulatory submissions may not occur or be submitted in a timely manner; the FDA may make adverse decisions regarding crinecerfont; crinecerfont may not be found to be safe and/or effective or may not prove to be beneficial to patients; development activities for crinecerfont may not be completed on time or at all; clinical development activities may be delayed for regulatory or other reasons, may not be successful or replicate previous and/or interim clinical trial results, or may not be predictive of real-world results or of results in subsequent clinical trials; competitive products and technological changes that may limit demand for our products; uncertainties relating to patent protection and intellectual property rights of third parties; our dependence on third parties for development and manufacturing activities related to crinecerfont, and our ability to manage these third parties; our future financial and operating performance; risks and uncertainties associated with the commercialization of our products; and other risks described in the Company's periodic reports filed with the Securities and Exchange Commission, including without limitation the Company's quarterly report on Form 10-Q for the quarter ended March 31, 2024. Neurocrine Biosciences disclaims any obligation to update the statements contained in this press release after the date hereof.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/neurocrine-biosciences-announces-publication-of-primary-cahtalyst-pediatric-phase-3-study-results-of-crinecerfont-for-the-treatment-of-cah-in-the-new-england-journal-of-medicine-302161432.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/neurocrine-biosciences-announces-publication-of-primary-cahtalyst-pediatric-phase-3-study-results-of-crinecerfont-for-the-treatment-of-cah-in-the-new-england-journal-of-medicine-302161432.html
SOURCE Neurocrine Biosciences, Inc.