CARsgen Announced 2023 Annual Results
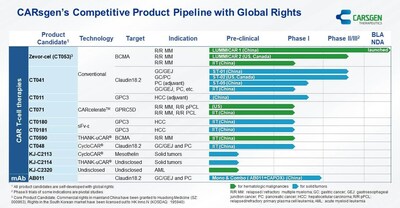
- CARsgen achieved NMPA approval for zevorcabtagene autoleucel (CT053) and satricabtagene autoleucel (CT041) IND for pancreatic cancer.
- Successful clinical trials with GPC3 CAR-T cells in hepatocellular carcinoma patients.
- FDA clearance for CT071 for multiple myeloma and primary plasma cell leukemia.
- Collaboration agreements with Huadong Medicine and Moderna for commercialization and research on CAR T-cell therapies.
- None.
Insights
The approval of CAR T-cell therapies, such as zevorcabtagene autoleucel for relapsed/refractory multiple myeloma, represents a significant advancement in the field of oncology. The conditional approval by the NMPA indicates a recognition of the therapy's potential, despite the need for further data to confirm its efficacy and safety. The clinical outcomes, such as the reported 7-year disease-free survival in two hepatocellular carcinoma patients treated with CAR-GPC3 T-cells, suggest a promising horizon for patients with limited treatment options. The collaboration between CARsgen and Huadong Medicine for the commercialization of zevorcabtagene autoleucel in China is also noteworthy, as it could enhance the therapy's accessibility to a broader patient population.
The progression of multiple CAR T-cell product candidates through various stages of clinical and regulatory development is an indicator of CARsgen's robust pipeline and potential for growth in the biotechnology sector. The proprietary CARcelerateTM platform's ability to shorten manufacturing times is particularly remarkable, as it could significantly reduce production costs and improve the scalability of CAR T-cell therapies. Such technological advancements are important for maintaining a competitive edge in the rapidly evolving landscape of cancer treatments. Furthermore, the collaborations with Moderna to investigate a combination therapy approach could lead to synergistic effects, potentially increasing the efficacy of the treatments and opening up new market opportunities.
The strategic partnerships and regulatory milestones achieved by CARsgen Therapeutics are likely to have a positive impact on investor sentiment and the company's stock market performance. The approval of new therapies and the advancement of trials into later stages typically serve as catalysts for stock valuation increases. The market for CAR T-cell therapies is expanding and CARsgen's positioning with multiple product candidates across different stages of development and cancer types could make it a significant player in the industry. Additionally, the collaborations with established companies such as Moderna and Huadong Medicine could provide financial and strategic benefits, enhancing CARsgen's market presence and potentially leading to an increase in market share.
Business Highlights
- Zevorcabtagene autoleucel (CT053) NDA was approved by the NMPA.
- Satricabtagene autoleucel (CT041) IND was approved by the NMPA for the postoperative adjuvant therapy of Claudin18.2 positive pancreatic cancer.
- CT011 IND was approved by the NMPA for GPC3-positive stage IIIa hepatocellular carcinoma at high risk of recurrence after surgical resection.
- Two hepatocellular carcinoma patients treated with a combination of local therapy and GPC3 CAR-T cells achieved disease-free survival exceeding 7 years.
- CT071 IND was cleared by the FDA for relapsed/refractory multiple myeloma and relapsed/refractory primary plasma cell leukemia.
- Developed a proprietary CARcelerateTM platform, shortening the manufacturing time to around 30 hours. The platform has been utilized for CT071.
- CARsgen and Huadong Medicine entered into a collaboration agreement for the commercialization of zevorcabtagene autoleucel in mainland
China . - CARsgen and Moderna initiated a collaboration agreement to investigate satricabtagene autoleucel in combination with an mRNA cancer vaccine.
Dr. Zonghai Li, Founder, Chairman of the Board, Chief Executive Officer, and Chief Scientific Officer of CARsgen Therapeutics, said, "In 2023, CARsgen remained dedicated to our vision, 'Making Cancer Curable' and were committed to reinforcing our team and improving operational efficiency. We made substantial progresses in the regulatory and clinical development of our innovative products and the advancement of new technology platforms. Multiple important milestones for different product candidates across clinical, regulatory, and business development were achieved. We are optimistic that we will navigate and overcome the challenges ahead with resilience and determination, advancing our innovative cell therapies."
Zevorcabtagene autoleucel (CT053) is an autologous fully human CAR T-cell product candidate against B-cell maturation antigen (BCMA) for the treatment of relapsed/refractory multiple myeloma (R/R MM). As informed by the NMPA on March 1, 2024, zevorcabtagene autoleucel was granted conditional approval on February 23, 2024 for the treatment of adult patients with relapsed or refractory multiple myeloma who have progressed after at least 3 prior lines of therapy (including a proteasome inhibitor and an immunomodulatory agent). An update from the Phase I study in
Satricabtagene autoleucel (CT041) is an autologous humanized CAR T-cell product candidate against Claudin18.2 (CLDN18.2), a membrane protein highly expressed in certain cancers. As of the date of the announcement, satricabtagene autoleucel, based on our information, is the world's first CAR T-cell candidate for the treatment of solid tumors entering a Phase II clinical trial. In April 2023, satricabtagene autoleucel IND was approved by the National Medical Products Administration (NMPA) for the postoperative adjuvant therapy of Claudin18.2 positive pancreatic cancer (PC) (CT041-ST-05, NCT05911217). In May 2023, the Phase 2 part of the Phase 1b/2 clinical trial (NCT04404595) in the
CT011 is an autologous CAR T-cell product candidate against Glypican-3 (GPC3). In January 2024, CT011 IND was approved by the NMPA for GPC3-positive stage IIIa hepatocellular carcinoma at high risk of recurrence after surgical resection.
In July 2023, an article titled "Combined local therapy and CAR-GPC3 T-cell therapy in advanced hepatocellular carcinoma: a proof-of-concept treatment strategy" was published in Cancer Communication (
CT071 is an autologous fully human CAR T-cell therapy candidate against G protein-coupled receptor class C group 5 member D (GPRC5D) developed utilizing CARsgen's proprietary CARcelerateTM platform for the treatment of R/R MM and relapsed/refractory primary plasma cell leukemia (R/R pPCL). The IND was cleared by the FDA on November 30, 2023 for R/R MM and R/R pPCL. An investigator-initiated trial (IIT) is ongoing in
In January 2023, CARsgen and Huadong Medicine (
In August 2023, CARsgen and Moderna, Inc. (Nasdaq: MRNA, "Moderna") have initiated a collaboration agreement to investigate satricabtagene autoleucel in combination with Moderna's investigational Claudin18.2 mRNA cancer vaccine.
About CARsgen Therapeutics Holdings Limited
CARsgen is a biopharmaceutical company with operations in
Forward-looking Statements
All statements in this press release that are not historical fact or that do not relate to present facts or current conditions are forward-looking statements. Such forward-looking statements express the Group's current views, projections, beliefs and expectations with respect to future events as of the date of this press release. Such forward-looking statements are based on a number of assumptions and factors beyond the Group's control. As a result, they are subject to significant risks and uncertainties, and actual events or results may differ materially from these forward-looking statements and the forward-looking events discussed in this press release might not occur. Such risks and uncertainties include, but are not limited to, those detailed under the heading "Principal Risks and Uncertainties" in our most recent annual report and interim report and other announcements and reports made available on our corporate website, https://www.carsgen.com. No representation or warranty is given as to the achievement or reasonableness of, and no reliance should be placed on, any projections, targets, estimates or forecasts contained in this press release.
Contact CARsgen
For more information, please visit https://www.carsgen.com/
Public Relations: PR@carsgen.com
Investor Relations: IR@carsgen.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/carsgen-announced-2023-annual-results-302100388.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/carsgen-announced-2023-annual-results-302100388.html
SOURCE CARsgen Therapeutics
FAQ
What were the highlights of CARsgen Therapeutics Holdings 's 2023 Annual Results?
What approvals did CARsgen receive from the NMPA?
What successful clinical trials did CARsgen conduct?
What FDA clearance did CT071 receive?