What Women Want: Accuracy is Critical to Future Wearable Use
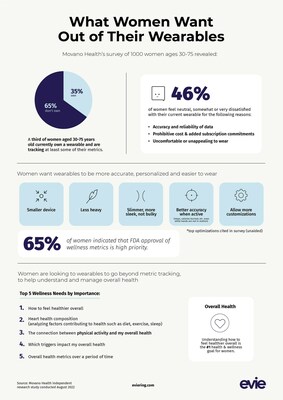
Movano Health's recent survey of 1,000 U.S. women highlights the importance of data accuracy in wearable health devices. With plans for FDA clearance of its Evie Ring, Movano aims to enhance consumer trust in health data. The survey revealed that 65% of participants prioritize FDA approval for wellness metrics, emphasizing the need for reliable data to communicate with healthcare providers. While many women are interested in wearables for health management, accuracy remains a key concern. Movano seeks to position itself in the market by providing highly accurate health insights to address these needs.
- Plans for FDA clearance of the Evie Ring could boost market credibility.
- Survey indicates strong interest in wearables for health management among women.
- 65% of women prioritize FDA approval, supporting the company's strategic focus.
- Concerns about accuracy could hinder the adoption of wearable devices.
Movano Health's survey of 1,000 U.S. women reveals that accuracy of data is a critical feature for those considering a wearable to help them manage health. The company is eyeing an FDA clearance submission for its Evie Ring to give consumers a higher level of trust in their health data.
PLEASANTON, Calif., March 7, 2023 /PRNewswire/ -- Movano Health (Nasdaq: MOVE), a purpose-driven healthcare solutions company at the intersection of medical and consumer devices, announces the results of their study of opportunities within the wearables market. The results speak clearly to accuracy being a major factor for women considering a wearable device.
Asked to evaluate nearly 15 "product performance" features,
Most women surveyed expressed they are interested in purchasing a wearable to focus on their overall health, but when asked what their hesitations were, the most common issue was related to accuracy. Until consistent accuracy is achieved, wearables will continue to be used with a focus on fitness performance rather than a true measure of overall health and wellness.
"The results of the study validated for us that FDA clearance is a necessary next step in the evolution of wearables and the management of overall health. We're hearing a consistent requirement from women that wearables be comfortable and monitor their vitals accurately, and that's best achieved with an FDA cleared, medical device," said John Mastrototaro, CEO of Movano Health.
Additionally, when women were asked which lifestyle attitudes and goals most resonated with them, most women said they are looking to understand their body (
The interviews included 1,000 women between the ages of 30-75, who were either current or prospective users of wearables and/or digital wellness tracking devices. The women participated in the survey in two phases. Phase one was conducted using a quantitative methodology consisting of a 20-minute online survey. Phase two was a three- day online discussion panel with a focused group using a qualitative methodology to develop a deeper understanding of their needs and how wearables play a role in their daily lives.
For more information on Movano Health, visit www.movanohealth.com.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/what-women-want-accuracy-is-critical-to-future-wearable-use-301763947.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/what-women-want-accuracy-is-critical-to-future-wearable-use-301763947.html
SOURCE Movano
FAQ
What does the Movano Health survey reveal about women's preferences for wearables?
What is the significance of FDA clearance for Movano's Evie Ring?
When was the survey by Movano Health conducted?
What age group was targeted in the Movano Health survey?