Monopar Announces Promising Preclinical Data for its MNPR-101 Radiopharma Program Targeting Advanced Cancers
- None.
- None.
Insights
The urokinase plasminogen activator receptor (uPAR) is a significant biomarker and therapeutic target for various aggressive cancers, including triple-negative breast, colorectal and pancreatic cancers. The preclinical data presented by Monopar Therapeutics for their MNPR-101 radiopharmaceutical program indicates a high specificity of the agent for tumors expressing uPAR. This specificity is crucial for minimizing damage to healthy tissue during radiopharmaceutical therapy, which is a common concern in oncology treatments. The ability to deliver higher doses to the tumor while reducing exposure to normal tissues could result in improved therapeutic outcomes and reduced side effects for patients.
Furthermore, the use of radioisotopes like actinium-225 (Ac-225) in conjugation with MNPR-101 is noteworthy. Ac-225 emits alpha particles, which have a high linear energy transfer, leading to potent cytotoxic effects within a short range. This property makes Ac-225 an attractive option for targeted cancer therapy, as it can destroy cancer cells while sparing surrounding healthy cells. The preliminary efficacy data showing near complete elimination of tumors in preclinical models is promising and suggests that if these results can be replicated in human trials, there could be a significant impact on the treatment landscape for these hard-to-treat cancers.
From a research and development perspective, the advancements Monopar Therapeutics is making in the radiopharmaceutical field are significant. The optimization of tumor uptake demonstrated in preclinical positron emission tomography (PET) imaging represents a notable achievement in drug development. PET imaging is a highly sensitive technique for detecting biochemical changes in the body and is widely used in oncology to assess tumor metabolism and monitor treatment response. Enhancing the uptake of MNPR-101-Zr in tumors could lead to more accurate and effective imaging, which is critical for staging cancer and tailoring treatment plans.
The transition from preclinical to clinical stages is a pivotal moment for any therapeutic candidate. The clearance to commence a Phase 1 dosimetry clinical trial in Australia for MNPR-101-Zr marks a crucial step for Monopar. This trial will evaluate the distribution, retention and safety of the radiopharmaceutical in humans, which is essential for determining the appropriate dosage for subsequent therapeutic efficacy trials. The outcome of this trial will be a key determinant of the program's future and its potential impact on the oncology sector.
The announcement by Monopar Therapeutics could have implications for the company's market position and investor interest, especially given the high unmet medical need in aggressive cancers like triple-negative breast, colorectal and pancreatic cancers. The market for cancer therapeutics is highly competitive and innovations that offer potential improvements in safety and efficacy can capture significant attention. If Monopar's MNPR-101 radiopharmaceutical program continues to show promising results in human trials, it could lead to increased partnership opportunities, funding and potentially expedited regulatory pathways.
Investors often look for indicators of a biopharmaceutical company's potential and promising preclinical data is one such indicator. However, it is important to note that the transition from preclinical to clinical success is not guaranteed and there are numerous regulatory, clinical and financial hurdles to overcome before a product can reach the market. Nonetheless, the company's progress and the upcoming presentation of results at a scientific meeting could generate buzz and possibly affect the stock's performance in the short term, as the market reacts to potential future value.
WILMETTE, Ill., Feb. 22, 2024 (GLOBE NEWSWIRE) -- Monopar Therapeutics Inc. (Nasdaq: MNPR), a clinical-stage biopharmaceutical company focused on developing innovative treatments for cancer patients, today announced promising preclinical imaging and therapeutic efficacy data for its MNPR-101 radiopharmaceutical program. This novel first-in-class radiopharma program targets cancers expressing the urokinase plasminogen activator receptor (uPAR), which include a majority of all triple-negative breast, colorectal, and pancreatic cancers.
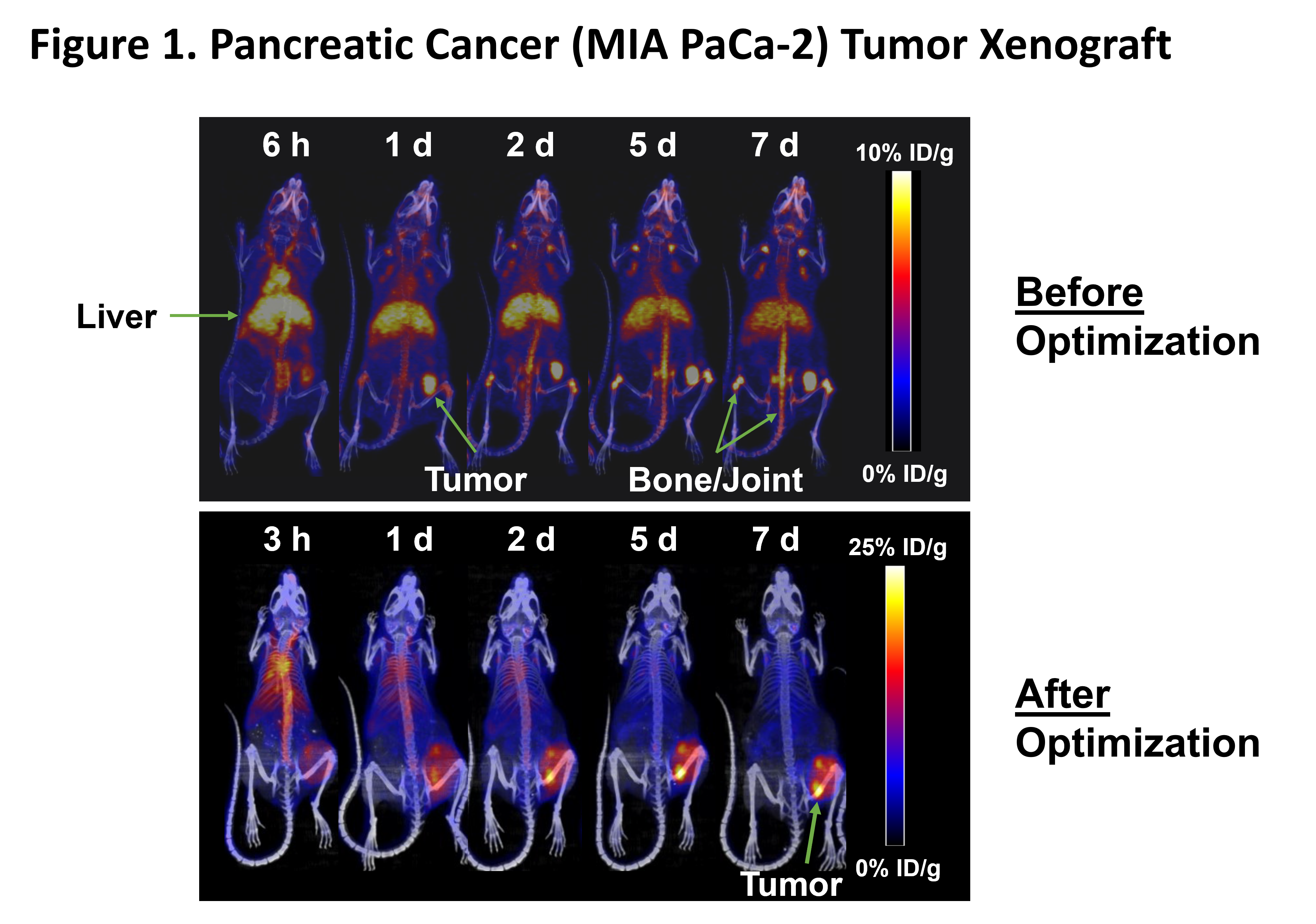
MNPR-101 Conjugated to Imaging Radioisotope
Maximizing the dose delivered to the tumor relative to normal tissue is of paramount importance in radiopharmaceutical therapy. Figure 1 below shows the before and after optimization of MNPR-101-Zr, Monopar’s radiopharmaceutical imaging agent for advanced solid tumors expressing uPAR. Monopar’s in-house radiopharmaceutical development team was able to significantly increase tumor uptake of MNPR-101-Zr while minimizing uptake in healthy tissue, as shown in this preclinical positron emission tomography (PET) sequential imaging time-series. The high specificity and durable tumor uptake are evident in the After Optimization panel below.
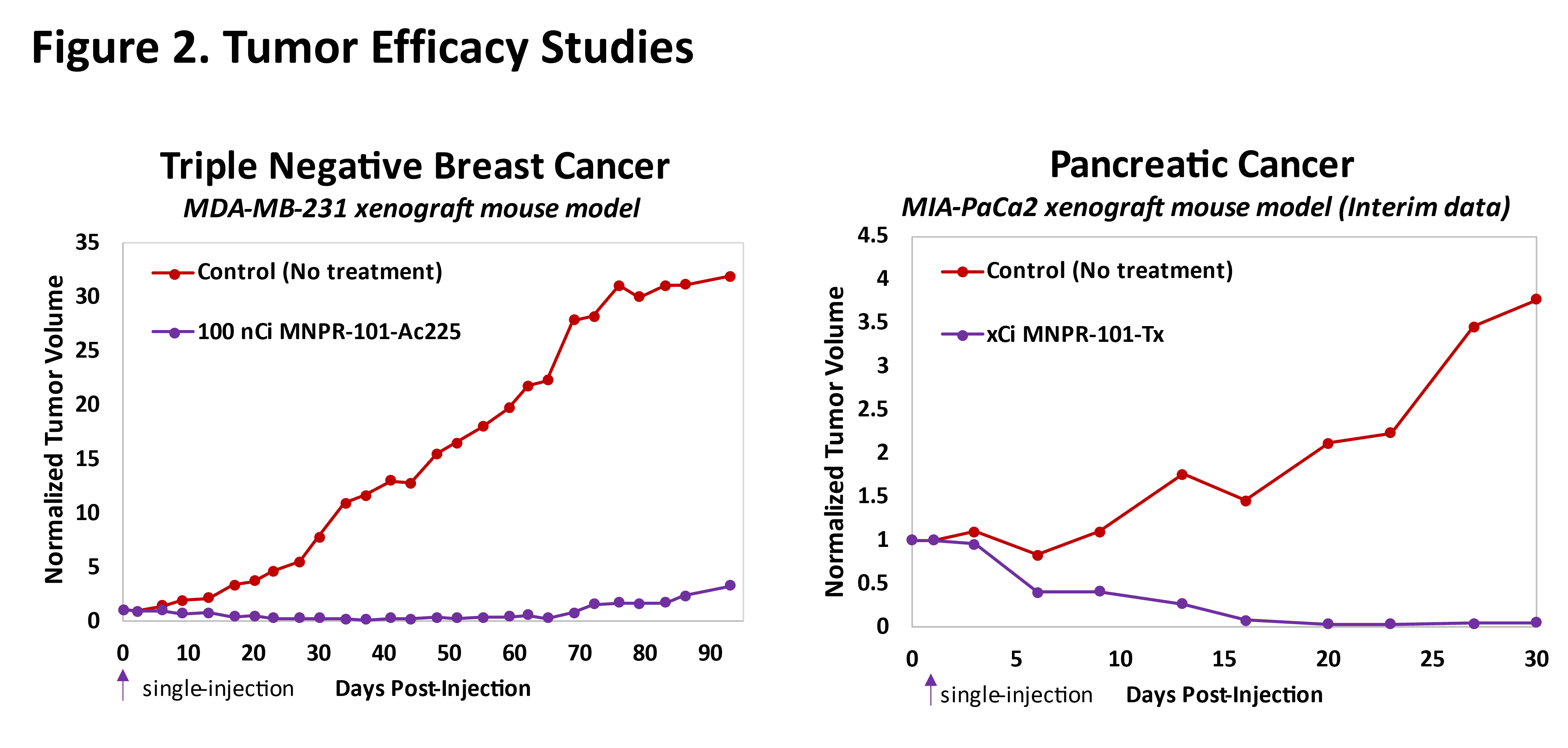
MNPR-101 Conjugated to Therapeutic Radioisotopes
Preclinical data to date demonstrate compelling and durable anti-tumor benefits with MNPR-101 conjugated to therapeutic radioisotopes. Figure 2 below shows preclinical efficacy data in a triple negative breast cancer as well as a pancreatic cancer human tumor xenograft mouse model utilizing two different therapeutic radioisotopes conjugated to MNPR-101; one of these radioisotopes has already been disclosed as being actinium-225 (Ac-225). The results in both show near complete elimination of the tumor after a single injection of the radiopharmaceutical agent. These studies demonstrate the potential of a MNPR-101 based radiopharmaceutical to provide a very meaningful clinical benefit to patients.
Monopar recently announced it received Human Research Ethics Committee (HREC) clearance in Australia to commence a Phase 1 dosimetry clinical trial for MNPR-101-Zr in advanced cancer patients. “As we prepare to launch this clinical trial, we are encouraged by the significant, precise, and durable accumulation we are seeing in tumors and the corresponding therapeutic benefit in preclinical human tumor xenograft models,” said Andrew Cittadine, Monopar’s Chief Operating Officer. “We are aiming to present these promising preclinical results at an upcoming scientific meeting.”
About Monopar Therapeutics Inc.
Monopar Therapeutics is a clinical-stage biopharmaceutical company focused on developing innovative treatments for cancer patients. Monopar's pipeline consists of Phase 1b-stage camsirubicin for the treatment of advanced soft tissue sarcoma; Phase 1-stage MNPR-101 for radiopharmaceutical use in advance cancers; and an early-stage camsirubicin analog, MNPR-202. For more information, visit: www.monopartx.com.
Forward-Looking Statements
Statements contained in this press release regarding matters that are not historical facts are "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. The words “may,” “will,” “could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “target” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Examples of these forward-looking statements include: that these studies demonstrate the potential of an MNPR-101 based radiopharmaceutical to provide a very meaningful clinical benefit to patients; and that we are aiming to present these promising preclinical results at an upcoming scientific meeting. The forward-looking statements involve risks and uncertainties including, but not limited to: that future preclinical or clinical data will not be as promising as the data to date; not initiating and enrolling the Phase 1 clinical trial; that MNPR-101-Zr may cause unexpected serious adverse effects or fail to image or be effective against the cancer tumors in humans; the potential for the HREC to put the Phase 1 trial on clinical hold at any time; and the significant general risks and uncertainties surrounding the research, development, regulatory approval, and commercialization of imaging agents and therapeutics. Actual results may differ materially from those expressed or implied by such forward-looking statements. Risks are described more fully in Monopar's filings with the Securities and Exchange Commission. All forward-looking statements contained in this press release speak only as of the date on which they were made. Monopar undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made. Any forward-looking statements contained in this press release represent Monopar’s views only as of the date hereof and should not be relied upon as representing its views as of any subsequent date.
CONTACT:
Monopar Therapeutics Inc.
Investor Relations
Kim R. Tsuchimoto
Chief Financial Officer
kimtsu@monopartx.com
Follow Monopar on social media for updates:
Twitter: @MonoparTx LinkedIn: Monopar Therapeutics
Photos accompanying this announcement are available at:
https://www.globenewswire.com/NewsRoom/AttachmentNg/3a5deb93-2122-4ee4-9ecb-745d9cbf923b
https://www.globenewswire.com/NewsRoom/AttachmentNg/cff0bf6c-68e7-440d-b721-5a5d77c28967

FAQ
What is the focus of Monopar Therapeutics Inc.'s (MNPR) MNPR-101 radiopharmaceutical program?
What is the significance of maximizing the dose delivered to the tumor relative to normal tissue in radiopharmaceutical therapy?
What did Monopar's in-house development team achieve with MNPR-101-Zr in preclinical imaging?
What did preclinical data demonstrate about MNPR-101 conjugated to therapeutic radioisotopes?







