Monopar Announces Positive Early Human Clinical Data Validating the Tumor Targeting Ability of MNPR-101-Zr
Rhea-AI Summary
Monopar Therapeutics Inc. (Nasdaq: MNPR) announced positive early data from its ongoing MNPR-101-Zr Phase 1 imaging and dosimetry clinical trial, validating the tumor targeting ability of MNPR-101-Zr in humans. The trial focuses on MNPR-101, a proprietary first-in-class humanized monoclonal antibody targeting cancers expressing the urokinase plasminogen activator receptor (uPAR).
Key findings include:
- Highly preferential uptake of MNPR-101-Zr in metastatic tumors relative to normal tissue
- Specificity and durability of MNPR-101-Zr in tumor targeting
- Alignment of MNPR-101-Zr uptake with previously observed metastatic tumors on FDG PET imaging
Monopar also received clearance in Australia to initiate an MNPR-101-Lu Phase 1 therapeutic clinical trial, scheduled for Q4 2024. Additional data will be presented at the European Association of Nuclear Medicine 2024 Annual Congress in October.
Positive
- Positive early data from MNPR-101-Zr Phase 1 trial confirming tumor targeting ability in humans
- Highly preferential uptake of MNPR-101-Zr in metastatic tumors relative to normal tissue
- MNPR-101-Zr uptake aligns with previously observed metastatic tumors on FDG PET imaging
- Clearance received in Australia to initiate MNPR-101-Lu Phase 1 therapeutic clinical trial
- Abstract accepted as 'Top-Rated Oral Presentation' at European Association of Nuclear Medicine 2024 Annual Congress
Negative
- None.
News Market Reaction 1 Alert
On the day this news was published, MNPR gained 64.58%, reflecting a significant positive market reaction.
Data tracked by StockTitan Argus on the day of publication.
WILMETTE, Ill., Sept. 12, 2024 (GLOBE NEWSWIRE) -- Monopar Therapeutics Inc. (Nasdaq: MNPR), a clinical-stage radiopharma company focused on developing innovative treatments for cancer patients, today announced positive early data from its ongoing open-label MNPR-101-Zr Phase 1 imaging and dosimetry clinical trial confirming MNPR-101-Zr’s tumor targeting ability in humans.
MNPR-101 is Monopar’s proprietary first-in-class humanized monoclonal antibody that targets cancers expressing the urokinase plasminogen activator receptor (uPAR). These include a majority of all triple-negative breast, colorectal, bladder, ovarian, gastric, and pancreatic cancers.
A total-body positron emission tomography (PET) image was taken at 168 hours (7 days) post administration of MNPR-101-Zr (a zirconium-89 imaging radioisotope conjugated to MNPR-101) of the first cancer patient in the trial with one of the known high uPAR-expressing cancer types. The results, seen in Figure 1, demonstrate the specificity, durability, and uptake of MNPR-101-Zr in the metastatic tumors relative to normal tissue. The regions of higher uptake also align with the locations of the previously observed metastatic tumors on conventional FDG PET imaging.
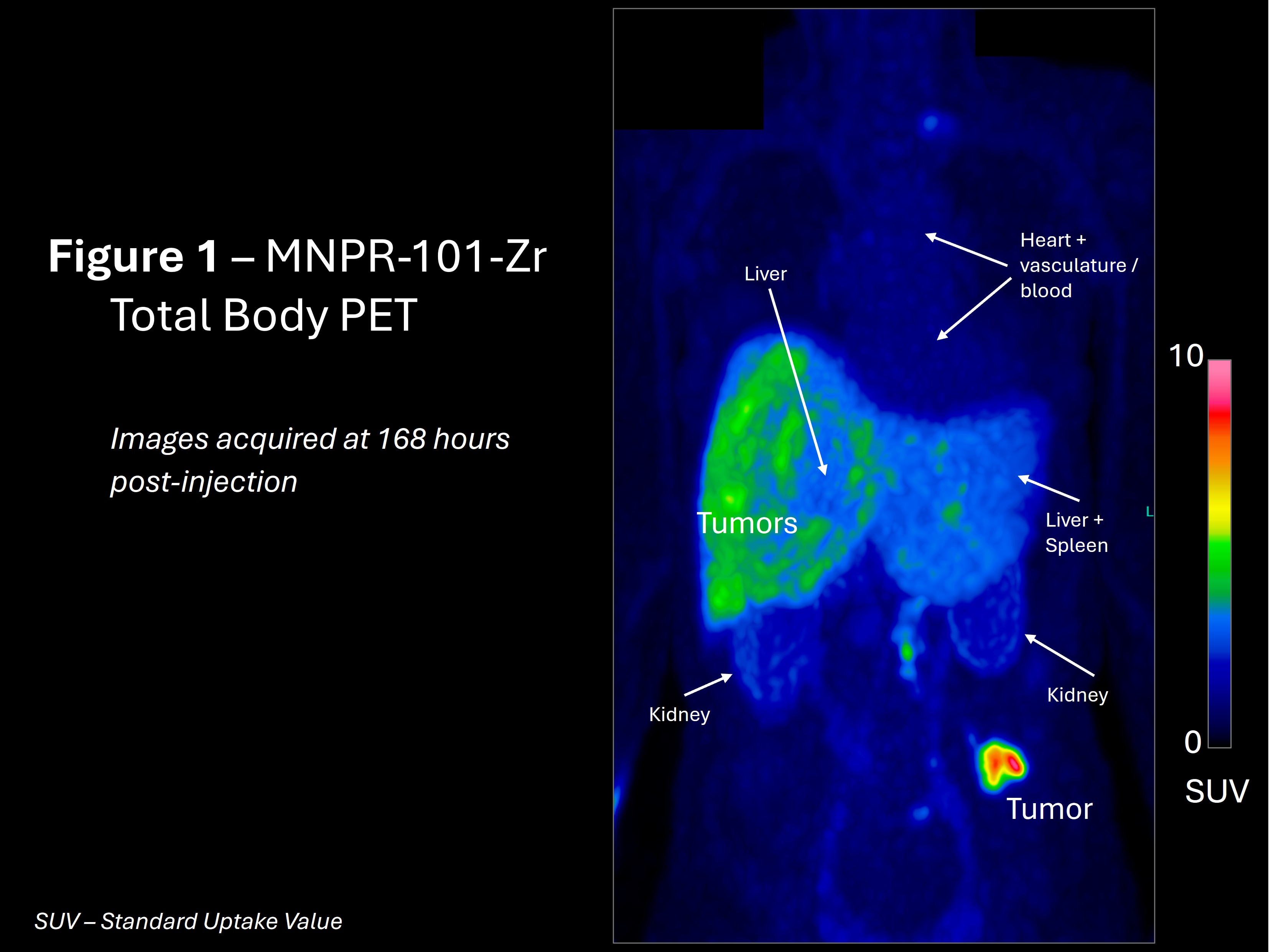
“This is exactly what we had hoped to see – highly preferential uptake in the tumor,” said Andrew Cittadine, Monopar’s Chief Operating Officer.
MNPR-101-Zr was evaluated against FDG, the gold standard for detecting metastatic tumors. Figure 2 shows FDG uptake in its highest-uptake tumor compared to MNPR-101-Zr uptake in the same tumor imaged on the same Siemens Biograph Vision Quadra™ PET/CT scanner.
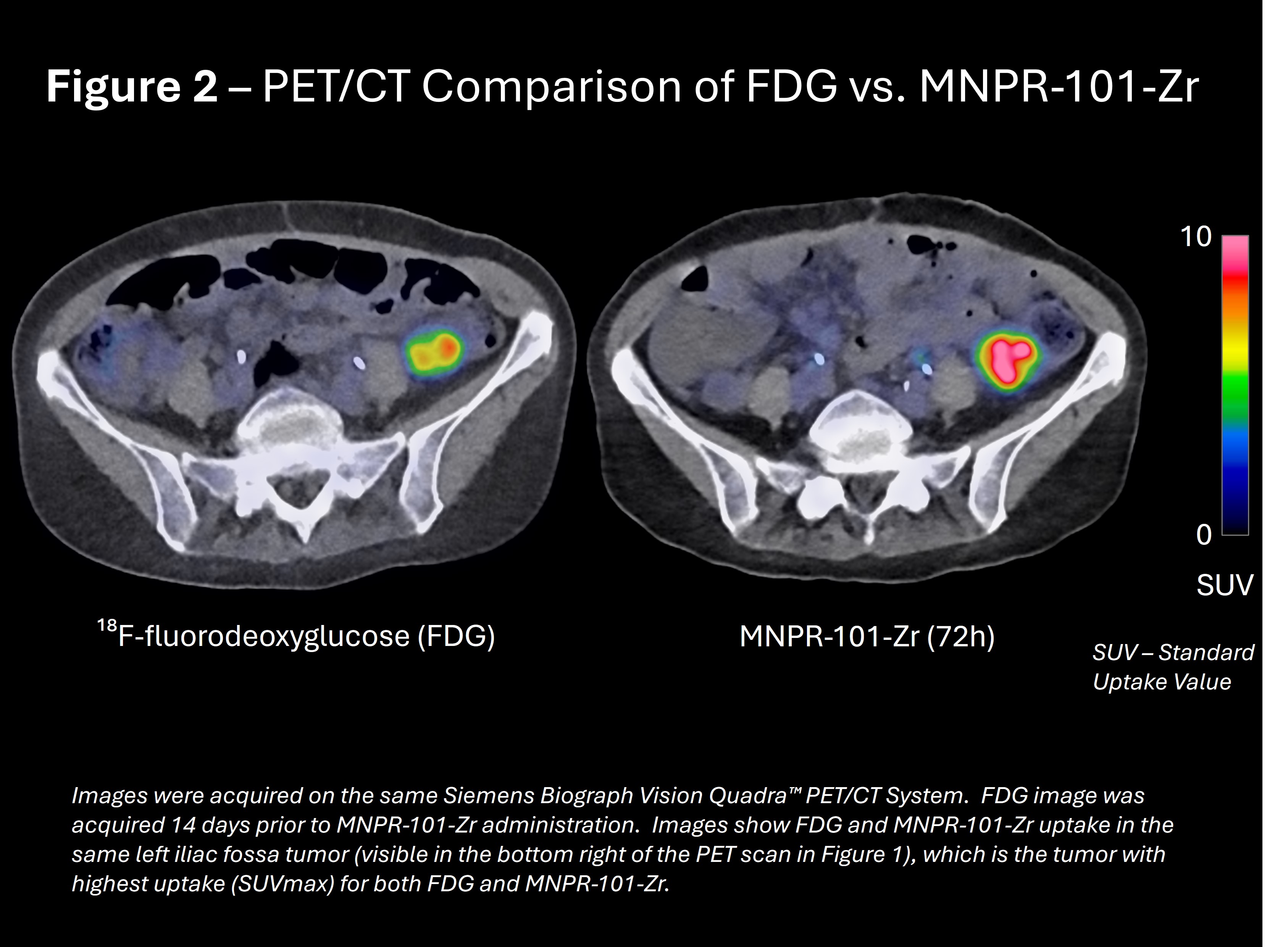
“At the Melbourne Theranostic Innovation Centre, we utilize one of the world's most sensitive PET/CT scanners. Using the same scanner for FDG and MNPR-101-Zr, the results show MNPR-101-Zr achieved uptake at sites of known disease with retention out to late points, which is promising for future therapeutic translation,” said Professor Rodney Hicks, MBBS(Hons), MD, FRACP, FICIS, FAAHMS, lead investigator on the MNPR-101-Zr Phase 1 imaging and dosimetry clinical trial.
Monopar recently received clearance in Australia to initiate an MNPR-101-Lu Phase 1 therapeutic clinical trial [link] which is currently scheduled to launch in the fourth quarter of this calendar year.
“We are looking forward to sharing additional data at the upcoming European Association of Nuclear Medicine 2024 Annual Congress to be held in Hamburg, Germany on October 19-23, 2024, where our abstract has been accepted as a 'Top-Rated Oral Presentation' within the Scientific Program,” said Chandler Robinson, MD, Monopar’s Chief Executive Officer.
Further information about the ongoing MNPR-101-Zr Phase 1 imaging and dosimetry clinical trial is available at www.ClinicalTrials.gov under study identifier NCT06337084.
About Monopar Therapeutics Inc.
Monopar Therapeutics is a clinical-stage radiopharmaceutical company focused on developing innovative treatments for cancer patients, including Phase 1-stage MNPR-101-Zr for imaging advanced cancers, Phase 1-stage MNPR-101-Lu and late preclinical-stage MNPR-101-Ac225 for the treatment of advanced cancers, as well as early development stage programs against solid cancers. For more information, visit: www.monopartx.com.
Forward-Looking Statements
Statements contained in this press release regarding matters that are not historical facts are "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. The words “may,” “will,” “could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “target” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Examples of these forward-looking statements include: the results show MNPR-101-Zr achieved uptake at sites of known disease with retention out to late points, which is promising for future therapeutic translation; and that an MNPR-101-Lu Phase 1 therapeutic clinical trial is currently scheduled to launch in the fourth quarter of this calendar year. The forward-looking statements involve risks and uncertainties including, but not limited to: that Monopar may not launch its MNPR-101-Lu therapeutic study in the fourth quarter of 2024, if at all; that the Phase 1 imaging and dosimetry clinical trial in advanced cancer patients with MNPR-101-Zr may not yield consistently satisfactory results; that future preclinical or clinical data may not be as promising as the data to date; that MNPR-101-Zr and/or MNPR-101-Lu may cause unexpected serious adverse effects or fail to be effective against the cancer tumors in humans; that Monopar may expend available funds sooner than anticipated or require additional funding due to change in circumstances or unanticipated events; and the significant general risks and uncertainties surrounding the research, development, regulatory approval, and commercialization of imaging agents and therapeutics. Actual results may differ materially from those expressed or implied by such forward-looking statements. Risks are described more fully in Monopar's filings with the Securities and Exchange Commission. All forward-looking statements contained in this press release speak only as of the date on which they were made. Monopar undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made. Any forward-looking statements contained in this press release represent Monopar’s views only as of the date hereof and should not be relied upon as representing its views as of any subsequent date.
CONTACT:
Monopar Therapeutics Inc.
Investor Relations
Karthik Radhakrishnan
Chief Financial Officer
karthik@monopartx.com
Follow Monopar on social media for updates:
Twitter: @MonoparTx LinkedIn: Monopar Therapeutics
Photos accompanying this announcement are available at:
https://www.globenewswire.com/NewsRoom/AttachmentNg/cda29080-7e74-46b0-8b93-5925a209f2ab
https://www.globenewswire.com/NewsRoom/AttachmentNg/cb0b4c63-91dc-46e1-a3c5-862ccc4251d8









