MannKind Announces Six-Month Results From Phase 3 INHALE-1 Pediatric Diabetes Trial Utilizing Inhaled Insulin (Afrezza®)
MannKind (MNKD) announced six-month results from its Phase 3 INHALE-1 study of Afrezza (inhaled insulin) in children and adolescents aged 4-17 years. The 26-week trial involved 230 subjects randomized to either Afrezza or multiple daily injections (MDI) of rapid-acting insulin.
The modified intent-to-treat (mITT) analysis, excluding one non-adherent patient, demonstrated non-inferiority of Afrezza to MDI with a between-group difference of 0.370% in HbA1c change, meeting the primary endpoint's threshold of 0.4%. No significant differences in lung function or safety parameters were observed between groups. The company plans to meet with FDA regarding a potential supplemental new drug application (sNDA) submission in first half of 2025.
MannKind (MNKD) ha annunciato i risultati a sei mesi del suo studio di Fase 3 INHALE-1 su Afrezza (insulina inalata) in bambini e adolescenti di età compresa tra 4 e 17 anni. La sperimentazione di 26 settimane ha coinvolto 230 soggetti randomizzati a ricevere Afrezza o iniezioni multiple giornaliere (MDI) di insulina a rapido assorbimento.
L'analisi dell'intenzione a trattare modificata (mITT), escludendo un paziente non aderente, ha dimostrato la non inferiorità di Afrezza rispetto a MDI, con una differenza tra i gruppi di 0.370% nel cambiamento dell'HbA1c, raggiungendo la soglia dell'endpoint primario del 0.4%. Non sono state osservate differenze significative nella funzionalità polmonare o nei parametri di sicurezza tra i gruppi. L'azienda prevede di incontrare la FDA riguardo a una potenziale domanda di approvazione di un farmaco supplementare (sNDA) nella prima metà del 2025.
MannKind (MNKD) anunció los resultados de seis meses de su estudio de Fase 3 INHALE-1 sobre Afrezza (insulina inhalada) en niños y adolescentes de 4 a 17 años. El ensayo de 26 semanas incluyó a 230 sujetos asignados al azar a recibir Afrezza o inyecciones diarias múltiples (MDI) de insulina de acción rápida.
El análisis de intención de tratar modificado (mITT), excluyendo a un paciente no adherente, demostró la no inferioridad de Afrezza en comparación con MDI, con una diferencia entre grupos del 0.370% en el cambio de HbA1c, cumpliendo con el umbral del punto final primario del 0.4%. No se observaron diferencias significativas en la función pulmonar ni en los parámetros de seguridad entre los grupos. La compañía planea reunirse con la FDA sobre una posible presentación de una solicitud de nuevo fármaco suplementario (sNDA) en la primera mitad de 2025.
MannKind (MNKD)는 4세에서 17세 사이의 어린이 및 청소년을 대상으로 한 Afrezza(흡입 인슐린)의 3상 INHALE-1 연구의 6개월 결과를 발표했습니다. 26주간의 시험에는 Afrezza 또는 다회 일일 주사(MDI)의 신속 작용 인슐린을 받도록 무작위 배정된 230명의 피험자가 포함되었습니다.
하나의 비순응 환자를 제외한 수정된 치료 의도(mITT) 분석은 Afrezza가 MDI에 대해 비열등하다는 것을 보여주었으며, HbA1c 변화에서 그룹 간의 차이는 0.370%로, 주요 평가 지표의 임계값인 0.4%를 충족하였습니다. 두 그룹 간의 폐 기능이나 안전성 매개변수에서 유의미한 차이는 관찰되지 않았습니다. 회사는 2025년 상반기 내에 잠재적인 보완 신약 신청(sNDA)에 대해 FDA와 만날 계획입니다.
MannKind (MNKD) a annoncé les résultats de six mois de son étude de Phase 3 INHALE-1 sur Afrezza (insuline inhalée) chez les enfants et adolescents âgés de 4 à 17 ans. L'essai de 26 semaines a impliqué 230 sujets randomisés pour recevoir soit Afrezza, soit des injections multiples quotidiennes (MDI) d'insuline à action rapide.
L'analyse modifiée de l'intention de traiter (mITT), excluant un patient non adhérent, a démontré la non-infériorité d'Afrezza par rapport à MDI avec une différence entre les groupes de 0,370% dans le changement de HbA1c, atteignant le seuil de l'objectif primaire de 0,4%. Aucune différence significative dans la fonction pulmonaire ou les paramètres de sécurité n'a été observée entre les groupes. L'entreprise prévoit de rencontrer la FDA concernant une éventuelle demande de nouveau médicament complémentaire (sNDA) au cours de la première moitié de 2025.
MannKind (MNKD) hat die Ergebnisse einer sechsmonatigen Phase-3-Studie (INHALE-1) zu Afrezza (inhalierbare Insulin) bei Kindern und Jugendlichen im Alter von 4 bis 17 Jahren bekannt gegeben. Die 26-wöchige Studie umfasste 230 Probanden, die entweder Afrezza oder mehrere tägliche Injektionen (MDI) von schnell wirkendem Insulin erhielten.
Die modifizierte Analyse der Behandlungsabsicht (mITT), bei der ein nicht einhaltender Patient ausgeschlossen wurde, zeigte die Nichtunterlegenheit von Afrezza gegenüber MDI mit einem Unterschied zwischen den Gruppen von 0,370% im HbA1c-Veränderung, wodurch die primäre Zielvorgabe von 0,4% erreicht wurde. Es wurden keine signifikanten Unterschiede in der Lungenfunktion oder in den Sicherheitsparametern zwischen den Gruppen beobachtet. Das Unternehmen plant, sich in der ersten Hälfte von 2025 mit der FDA über einen möglichen Antrag auf eine ergänzende neue Arzneimittelzulassung (sNDA) zu treffen.
- None.
- None.
Insights
- Company plans to meet with FDA regarding potential sNDA submission in 1H 2025
- Call planned today at 8:30 a.m. (ET) to discuss company’s diabetes program progression
DANBURY, Conn. and WESTLAKE VILLAGE, Calif., Dec. 16, 2024 (GLOBE NEWSWIRE) -- MannKind Corporation (Nasdaq: MNKD), a company focused on the development and commercialization of inhaled therapeutic products and devices for patients with endocrine and orphan lung diseases, today announced six-month results from its Phase 3 INHALE-1 study of Afrezza (insulin human) Inhalation Powder in children and adolescents (aged 4-17 years of age). MannKind expects to submit a request for a supplemental new drug application (sNDA) meeting with the U.S. Food and Drug Administration (FDA) in 1H 2025 to discuss the data and filing timeline.
The INHALE-1 study is a 26-week, open-label clinical trial that randomized 230 subjects to one of two groups: Afrezza or multiple daily injections (MDI) of rapid acting insulin analog (RAA) in combination with basal insulin. The primary endpoint was a non-inferior change in HbA1c levels after 26 weeks. A 26-week extension phase in which all remaining MDI patients switched to Afrezza is still ongoing.
Results were as follows:
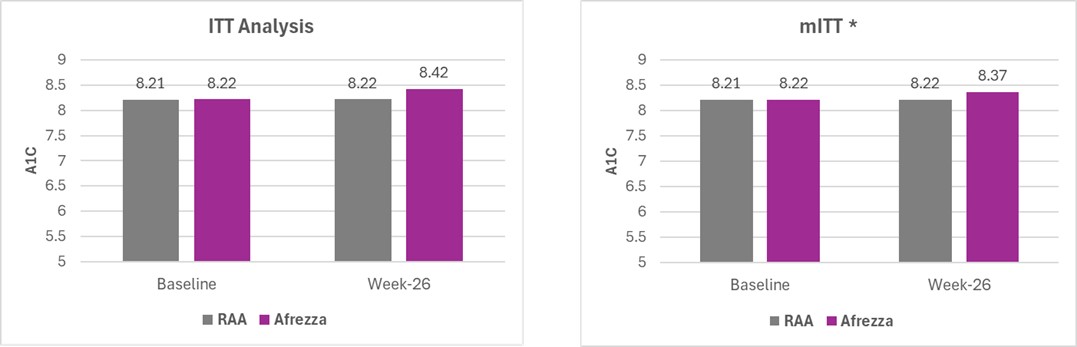
* mITT analysis excludes one outlier from the primary ITT endpoint who failed to adhere to the study protocol
An analysis of the full intent-to-treat population (ITT) found that the between-group difference in mean HbA1c change over 26 weeks exceeded the prespecified non-inferiority margin of
Over 26 weeks of treatment, no difference in lung function parameters were seen between the treatment groups. The Afrezza-treated patients had a mean FEV1 of 2.901 liters (
“The overall efficacy and safety outcomes seen in the first 26 weeks are encouraging. This represents a monumental step in our more than 25-year history of pioneering the development of inhaled insulin and working to bring this new treatment option to children and adolescents over the past seven years,” said Dr. Kevin Kaiserman, Senior Vice President, Therapeutic Area Head, Endocrine Diseases for MannKind Corporation.
“It was exciting to partner with MannKind and help lead this study to potentially expand the use of inhaled insulin, which is currently used successfully by many adults with diabetes, to a population that hasn’t had a treatment option other than injectable insulin in the history of their care,” said Dr. Roy W. Beck, founder of the Jaeb Center for Health Research who provided oversight for INHALE-1. “The six-month results are clinically meaningful and show Afrezza as a potential future treatment option for a growing pediatric population living with type 1 and type 2 diabetes.”
Conference Call
MannKind will host a live audio webcast beginning at 8:30 a.m. Eastern Time on Monday, December 16, 2024, to share results and discuss the company’s diabetes program progression. Participating in the conference call from MannKind will be Chief Executive Officer Michael Castagna, PharmD and Dr. Kaiserman. The webcast will be accessible via a link on MannKind’s website at https://investors.mannkindcorp.com/events-and-presentations. A replay will also be available in the same location within 24 hours following the call and be accessible for approximately 90 days.
About Afrezza
Afrezza (insulin human) Inhalation Powder is a rapid-acting inhaled human insulin indicated to improve glycemic control in adults with diabetes mellitus.
Limitations of Use: Not recommended for the treatment of diabetic ketoacidosis or in patients that smoke or have recently stopped smoking.
Important Safety Information
WARNING: RISK OF ACUTE BRONCHOSPASM IN PATIENTS WITH CHRONIC LUNG DISEASE
- Acute bronchospasm has been observed in Afrezza-treated patients with asthma and COPD
- Afrezza is contraindicated in patients with chronic lung disease such as asthma or COPD
- Before initiating Afrezza, perform a detailed medical history, physical examination, and spirometry (FEV1) to identify potential lung disease in all patients.
Most common adverse reactions are hypoglycemia, cough, and throat pain or irritation.
Please see additional Important Safety Information, Full Prescribing Information, including BOXED WARNING, available on Afrezza.com/safety.
About MannKind
MannKind Corporation (Nasdaq: MNKD) focuses on the development and commercialization of innovative inhaled therapeutic products and devices to address serious unmet medical needs for those living with endocrine and orphan lung diseases.
We are committed to using our formulation capabilities and device engineering prowess to lessen the burden of diseases such as diabetes, nontuberculous mycobacterial (NTM) lung disease, pulmonary fibrosis, and pulmonary hypertension. Our signature technologies – dry-powder formulations and inhalation devices – offer rapid and convenient delivery of medicines to the deep lung where they can exert an effect locally or enter the systemic circulation, depending on the target indication.
With a passionate team of Mannitarians collaborating nationwide, we are on a mission to give people control of their health and the freedom to live life.
Please visit mannkindcorp.com to learn more, and follow us on LinkedIn, Facebook, X or Instagram.
Forward-Looking Statements
This press release contains forward-looking statements about a planned meeting with the FDA, a potential sNDA submission and the potential expanded use of Afrezza that involves risks and uncertainties. Words such as “believes”, “anticipates”, “plans”, “expects”, “intends”, “will”, “goal”, “potential” and similar expressions are intended to identify forward-looking statements. These forward-looking statements are based upon MannKind’s current expectations. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation, the risk that issues that develop in the review by the FDA may subject us to unanticipated delays or prevent us from obtaining the expanded indication as well as other risks detailed in MannKind’s filings with the Securities and Exchange Commission, including under the “Risk Factors” heading of its Annual Report on Form 10-K for the year ended December 31, 2023, and subsequent periodic reports on Form 10-Q and current reports on Form 8-K. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date of this press release. All forward-looking statements are qualified in their entirety by this cautionary statement, and MannKind undertakes no obligation to revise or update any forward-looking statements to reflect events or circumstances after the date of this press release.
AFREZZA and MANNKIND are registered trademarks of MannKind Corporation.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/12f4dac8-7936-41b3-a2ad-7eef85fe3712








