Six-Year Natural History Comparison with Mirum’s LIVMARLI (maralixibat) Demonstrates Event-Free Survival and Transplant-Free Survival in Patients with Alagille Syndrome
Mirum Pharmaceuticals, Inc. (NASDAQ: MIRM) presented a new analysis of its drug LIVMARLI™ (maralixibat) at The Liver Meeting®, showing statistically significant improvements in six-year event-free survival and transplant-free survival for patients with Alagille syndrome (ALGS) (p<0.0001). The study, conducted by the Global Alagille Alliance (GALA), compared LIVMARLI's clinical trial data against a natural history cohort, indicating a 70% reduction in adverse clinical outcomes. These results support LIVMARLI's potential as a significant treatment for ALGS patients, especially in delaying liver transplants.
- LIVMARLI demonstrated a statistically significant improvement in six-year event-free survival (p<0.0001), with a 70% reduction in clinical outcomes for ALGS patients.
- Statistically significant transplant-free survival improvements were also observed (p<0.0001).
- The analysis was recognized at The Liver Meeting® as part of the 'Best of the Liver Meeting' series.
- None.
Insights
Analyzing...
- Results demonstrate statistically significant improvement in six-year event-free survival (p<0.0001) and transplant-free survival (p<0.0001)
- Study presented by
- Data underscore LIVMARLI’s potential to delay liver transplant and improve disease outcomes associated with Alagille syndrome
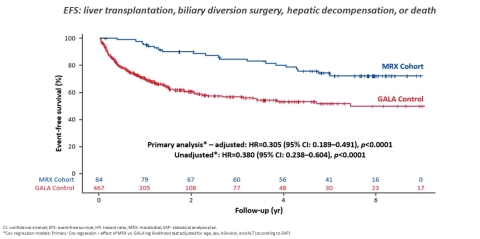
Significant Improvement in Event-Free Survival in Patients with ALGS with LIVMARLI Compared to Untreated Cohort (Graphic: Business Wire)
The oral, late-breaking session, which was selected as part of the “Best of the Liver Meeting” series, reported on an analysis independently conducted and presented by Dr.
The analysis demonstrated a highly significant improvement in six-year event-free survival with a p-value of <0.0001 (HR: 0.305,
“The extensive real-world analysis conducted by GALA validates the statistical robustness of the findings and suggest that patients who are treated with LIVMARLI may experience statistically significant event-free survival and transplant-free survival versus the natural history cohort,” said
“Cholestatic pruritus associated with Alagille syndrome has a dramatic and debilitating impact on the lives of patients and is a leading indication for liver transplantation,” said
Also at the meeting, a second late-breaker presentation was given by Dr.
To learn more about the data presented during the meeting, please visit the Alagille syndrome section of our Publications & Presentations page on Mirum’s website.
The results of the natural history comparison analysis were included in Mirum’s recent marketing authorization application submission (MAA) to the
About LIVMARLI™ (maralixibat) oral solution
LIVMARLI™ (maralixibat) oral solution is an orally administered, once-daily, ileal bile acid transporter (IBAT) inhibitor approved by the
LIVMARLI is currently being evaluated in late-stage clinical studies in other rare cholestatic liver diseases including progressive familial intrahepatic cholestasis and biliary atresia. LIVMARLI has received Breakthrough Therapy designation for ALGS and PFIC type 2 and orphan designation for ALGS, PFIC and biliary atresia. To learn more about ongoing clinical trials with LIVMARLI, please visit Mirum’s clinical trials section on the company’s website.
About Alagille syndrome
Alagille syndrome (ALGS) is a rare genetic disorder in which bile ducts are abnormally narrow, malformed and reduced in number, which leads to bile accumulation in the liver and ultimately progressive liver disease. The estimated incidence of ALGS is one in every 30,000 people.1 In patients with ALGS, multiple organ systems may be affected by the mutation, including the liver, heart, kidneys and central nervous system.2 The accumulation of bile acids prevents the liver from working properly to eliminate waste from the bloodstream and, according to recent reports,
IMPORTANT SAFETY INFORMATION
LIVMARLI can cause serious side effects, including:
Changes in liver tests. Changes in certain liver tests are common in patients with Alagille syndrome and can worsen during treatment with LIVMARLI. These changes may be a sign of liver injury and can be serious. Your healthcare provider should do blood tests before starting and during treatment to check your liver function. Tell your healthcare provider right away if you get any signs or symptoms of liver problems, including nausea or vomiting, skin or the white part of the eye turns yellow, dark or brown urine, pain on the right side of the stomach (abdomen) or loss of appetite.
Stomach and intestinal (gastrointestinal) problems. LIVMARLI can cause stomach and intestinal problems, including diarrhea, stomach pain, and vomiting during treatment. Tell your healthcare provider right away if you have any of these symptoms more often or more severely than normal for you.
A condition called Fat Soluble Vitamin (FSV) Deficiency caused by low levels of certain vitamins (vitamin A, D, E, and K) stored in body fat. FSV deficiency is common in patients with Alagille syndrome but may worsen during treatment. Your healthcare provider should do blood tests before starting and during treatment.
Other common side effects reported during treatment were bone fractures and gastrointestinal bleeding.
About the
Launched in
About
Mirum’s late-stage pipeline includes two investigational treatments for debilitating liver diseases affecting children and adults. Maralixibat (LIVMARLI), an oral ileal bile acid transporter (IBAT) inhibitor, is currently being evaluated in clinical trials for pediatric liver diseases and includes the MARCH Phase 3 study for progressive familial intrahepatic cholestasis (PFIC) and the EMBARK Phase 2b study for patients with biliary atresia. In addition, Mirum has an expanded access program open in
Mirum has submitted a Marketing Authorization Application to the
Mirum’s second investigational treatment, volixibat, also an oral IBAT inhibitor, is being evaluated in two registrational studies including the OHANA Phase 2b study for pregnant women with intrahepatic cholestasis of pregnancy and the VISTAS Phase 2b study for adults with primary sclerosing cholangitis. Mirum is planning to launch a Phase 2b study in primary biliary cholangitis later this year.
To augment its pipeline in cholestatic liver disease, Mirum has acquired the exclusive option to develop and commercialize gene therapy programs VTX-803 and VTX-802 for PFIC3 and PFIC2, respectively, from Vivet Therapeutics SAS, following preclinical evaluation and investigational new drug-enabling studies.
Follow Mirum on Twitter, Facebook, LinkedIn and Instagram.
Forward-Looking Statements
This press release includes forward-looking statements pertaining to the Company’s planned participation at a scientific conference, which may include discussion of the Company’s product candidates and technologies, and the therapeutic potential thereof. Such forward-looking statements are subject to risks and uncertainties that could cause actual results to differ materially from those expressed or implied in such statements. Applicable risks and uncertainties include those relating to our preclinical research and clinical programs and other risks identified under the heading “Risk Factors” included in our most recent Form 10-Q and Form 10-K filings and in other future filings with the
The Liver Meeting® is a registered trademark of the
1Danks, et al. Archives of Disease in Childhood 1977
2
3Vandriel, et al. GALA EASL 2020; Kamath, et al.
4Elisofon, et al.
View source version on businesswire.com: https://www.businesswire.com/news/home/20211115006047/en/
Media:
media@mirumpharma.com
Investors:
ir@mirumpharma.com
ir@mirumpharma.com
Source:







